- Operating principle
- ACE inhibitors
- The benefits of inhibitor drugs
- Classification and forms
- Application
- Contraindications for treatment with inhibitors
The group of inhibitors includes substances that slow down or completely block chemical (especially enzymatic) and physiological processes.
The name comes from the Latin word inhibere, which means “to capture.” One of the groups - ACE inhibitors - is used to treat pathologies of the cardiovascular system. Among the well-known ACE inhibitors is the drug Enalapril, used for heart failure, essential and renovascular hypertension, as well as for the prevention of the development of pathologies.
ACE inhibitors
ACE inhibitors, an angiotensin-converting enzyme, have been used in the treatment of diseases of the cardiovascular system for more than 30 years.
Angiotensin-converting enzyme is one of the key elements of the blood pressure regulation system. Under their influence, the inactive hormone angiotensin I is converted into angiotensin II, which has a vasoconstrictor effect. When using ACE inhibitors, its effect is suppressed, due to which the drugs of the group are able to lower blood pressure and are effective in the treatment of hypertension.
In addition, due to the release of nitric oxide and prostaglandin I2, drugs in this group inhibit the breakdown of the vasodilator (a substance that greatly dilates blood vessels) bradykinin.
For the treatment of heart failure, inhibitors are usually prescribed in doses that do not lower blood pressure.
The drugs are also prescribed to treat kidney failure.
The benefits of inhibitor drugs
ACE inhibitors:
- have an anti-inflammatory effect;
- promote urine excretion;
- increase the sensitivity of peripheral tissues to insulin;
- have a nephroprotective effect, that is, they help preserve kidney function;
- strengthens blood vessels: improves endothelial function, increases the elasticity of walls, is a preventive measure against damage to atherosclerotic plaque;
- prevent an increase in the size of the left ventricle of the heart;
- help to equalize heart rate;
- improve blood circulation in vital systems (parts of the central nervous system, kidneys, heart);
Classification and forms
ACE inhibitors are available in two types:
- drugs in active form;
- prodrugs are substances that become active after transformation in the gastrointestinal tract and kidneys.
Substances in this group are lipophilic, that is, they are eliminated through renal excretion (through the urinary system). For this reason, people with renal failure are recommended to start therapy with a reduced dosage of inhibitors. Among the inhibitors there are also hydrophilic drugs that are excreted in bile and feces.
ACE inhibitors, which are eliminated from the body through the liver, are considered the safest for long-term use. Drugs of the hydrophilic group are not metabolized in the body and are excreted in their original form, lipophilic inhibitors undergo partial metabolization and form organic substances, some of which are biologically active.
According to the duration of action, inhibitors are:
- short-acting, regular intake – 2-3 times a day;
- long-acting, regular use – 1-2 times a day.
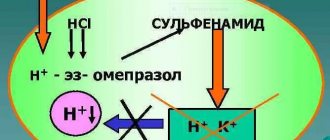
Mechanism of action
After entering the lumen of the stomach, proton pump inhibitors penetrate into the cavity of the small intestine, after which they dissolve, are absorbed into the systemic circulation and are transported through the bloodstream to the liver. Further, the active components of the drugs interact with the parietal (secretory) cells of the gastric mucosa.
During complex eradication of Helicobacter Pylori, before starting therapy, it is important to have pH measurements. For gastritis with zero acidity (achlorhydria), this group of drugs is not used, since complete atrophy of the gastric mucosa is observed, and the cells of the organ do not produce hydrochloric acid.
Application
ACE inhibitors are widely used in cardiology. The drugs of this group are used to treat:
- left ventricular systolic dysfunction regardless of the presence of chronic heart failure;
- left ventricular hypertrophy;
- arterial hypertension, including symptomatic;
- atherosclerosis of the carotid arteries;
- metabolic syndrome.
For preventive purposes, inhibitors are prescribed to patients who have suffered a myocardial infarction.
ACE inhibitors are prescribed to all patients with chronic heart failure, regardless of the stage and etiology of the disease. Initially, doctors prescribe minimal doses of drugs, gradually increasing them to the target.
For hypertension (arterial hypertension), inhibitors are used as an independent therapeutic agent and in combination with other drugs.
According to statistics, diabetes mellitus develops less frequently when treated with ACE inhibitors than when treated with diuretics and β-blockers.
In neurology, drugs from this group are used for primary and secondary prevention of cerebral stroke. Indications for prescribing inhibitors are also transient ischemic attack and stroke. In this case, the gender, age of the patient and initial blood pressure indicators do not matter. Treatment with ACE inhibitors leads to a reduction in the risk of a recurrent attack and the overall risk of complications on the cardiovascular system.
The group's drugs are effective for the treatment of endocrinological diseases: diabetic nephropathy, diabetes mellitus. Inhibitors are indicated for patients with obesity and insulin resistance because they increase sensitivity to this hormone. In addition, the drugs slow down the development of renal failure against the background of type I and II diabetes, and reduce the incidence of complications on the cardiovascular system.
Low molecular weight "crackers"
Irreversible covalent inhibitors are relatively low-active molecules that are transformed by a target enzyme into a highly active form that can irreversibly inhibit (i.e., “turn off”) the enzyme [1]. Let's imagine an ideal situation: we have developed an inhibitor that has absolutely no activity outside our target, but, having hit the target, recognizes it and turns it off, dooming it to death. But how can we make the inhibitor work only in the protein we need? The answer lies on the surface: to use the molecular target’s own mechanism of action against itself. The enzyme must first mistake the inhibitor for a familiar natural molecule. Then begin a reaction due to which the drug irreversibly binds to the target [2]. That is, coming as a harmless substrate, this “saboteur” tightly grabs the unsuspecting enzyme, depriving it of its ability to function and dooming it to certain death [3]! These substances seem to “hack” normal enzymatic reactions to turn off protein. A properly designed irreversible inhibitor is specific to a particular enzyme and is not harmful to other molecules. Therefore, drugs based on this approach may have the important advantage of few unwanted effects [1].
“What is the secret of such a strong binding of these irreversible inhibitors of yours?”
- you ask. In a covalent bond. There are several mechanisms by which drugs bind to their molecular targets. Typically, binding occurs through weak interactions [4]. A covalent bond is the strongest possible interaction and exceeds any other by tens or even hundreds of times (Table 1) [5]! Table 1. Types of interactions between inhibitor and receptor. [5]
| Communication type | Energy, kcal/mol |
| Covalent | 50–150 |
| Electrostatic | 5–10 |
| Hydrogen | 2–5 |
| Hydrophobic | 0,5–1 |
The mechanism of formation of a covalent bond is based on the fact that a pair of electrons of one of the atoms of the molecular target (nucleophile) is distributed between it and one of the inhibitor atoms (electrophile) (Fig. 1). Due to the high bond strength, the inhibitor is irreversibly attached to the enzyme and leads to the destruction of the latter. Restoration of the enzyme function occurs only after the synthesis of its new copies [5].
Figure 1. General mechanism of action of irreversible inhibitors.
[5]
What do these electrophilic groups that are used to create covalent irreversible inhibitors look like? You can find the answer by looking at Figure 2. The presence of one of these fragments in the structure of the drug may indicate that it acts according to a mechanism already known to you [6].
Figure 2. Examples of electrophilic groups found in irreversible inhibitors.
[6]
Despite the fact that the binding of irreversible inhibitors leads to the death of the enzyme, one should not think that one dose of such a drug can completely destroy the enzyme in the body. The presence and operation of genes will allow the creation of new copies of it, even if this takes from several hours to a couple of days [2].
Like everything in the world, covalent irreversible inhibitors have their advantages and disadvantages, which we presented in the format of Table 2 [6].
Table 2. Advantages and disadvantages of covalent irreversible inhibitors. [6]
| Advantages | Flaws |
| High efficiency and selectivity allow the use of smaller doses and reduce the risk of side effects | High activity of some representatives can lead to liver necrosis, mutations and even cancer! |
| Binding to important enzyme residues prevents the development of microbial resistance, which is important in the treatment of infections | Potential immunogenicity, as the resulting reaction product may cause an allergic response |
| Long-lasting action, since enzyme activity reappears only with the synthesis of its new copies |
Contraindications for treatment with inhibitors
Despite the general positive effect on the body, inhibitors have a number of contraindications in which treatment with this group of drugs is unacceptable. This:
- bilateral renal artery stenoses;
- severe renal failure;
- previous kidney transplant;
- severe aortic stenosis;
- pulmonary heart failure;
- childhood;
- individual intolerance;
- blood diseases;
- liver cirrhosis, hepatitis.
Also, the drugs are not suitable for pregnant and lactating women.
Inhibitors should be taken strictly as prescribed by the treating specialist. Uncontrolled use of drugs and self-medication lead to complications and the development of life-threatening pathologies.
The rocky road to developing effective drugs
If you think that developing irreversible covalent inhibitors is easy, then you are deeply mistaken. Even if we close our eyes to the fact that the development of any drug is an extremely labor-intensive and time-consuming process [7], the task will not be much simplified - after all, irreversible inhibitors require a special approach to their person. Today, not many approved drugs work in this way. And the mechanism of action of many of them was discovered only years after their development! Scientists along this path face a large number of sometimes insoluble problems.
One of them is the participation of one enzyme in several metabolic cascades at once. The drugs under discussion bind irreversibly to the target enzyme, destroying it for the time it takes to synthesize new copies of the enzyme. This process can take up to several days! If the target enzyme performed several functions, this is fraught with serious side effects. To circumvent the problem, irreversibly binding inhibitors are most often used to block one of the enzymes of viruses, bacteria, etc. that are potentially dangerous to humans [3].
The second problem is that, with low selectivity, irreversible inhibitors may bind to undesirable targets similar to the one we selected for inhibition. This can also lead to serious side effects. Therefore, an important task when optimizing the structure of inhibitors is to increase selectivity to their target [3].
Concerns about the side effects of irreversible inhibitors are not unfounded. The boom in persecution of this group of drugs was caused by research in the 1970s. Then it was found that two of the most widely known representatives of this group - paracetamol and furosemide - are highly toxic due to the fact that, when metabolized in the liver, they form active derivatives that form covalent bonds with its proteins [2], [8]. As it turned out later, these isolated cases are not related to many drugs from the group of irreversible inhibitors, and paracetamol and furosemide are still actively used in clinical practice.
Another common example of unwanted irreversible inhibition is cytochrome P450. Cytochromes are a group of enzymes in our body whose main task is to neutralize potentially dangerous substances. Alkenes and alkynes became the first irreversible inhibitors of cytochrome. As a result of inhibition of this enzyme, detoxification processes become impossible. A partial list of drugs that can cause this reaction includes 17α-alkenyl steroids, chloramphenicol, cyclophosphamide, spironolactone, furocoumarins, nicotine, isoniazid, and barbiturates. Sounds dangerous, doesn't it? Not much more dangerous than drinking grapefruit juice! Its components are capable of irreversibly binding to intestinal cytochrome and depriving this enzyme of activity for 24 hours! What could this lead to? Moreover, the medications taken at this time will enter our body more effectively, and an increase in their concentration increases the risk of developing undesirable effects [5]. This is why doctors recommend taking tablets only with water.
But can irreversible inhibitors always be considered safe drugs? Of course not. The search for irreversible inhibitors, indeed, does not always turn out to be successful for researchers. In particular, studies of arsenic-containing organic compounds for the treatment of trypanosomiasis ended in complete failure [9]. These compounds irreversibly inhibited the vital trypanosome enzymes due to the formation of bonds between the arsenic atom in the composition of the substances under study and the sulfur-containing residues of the enzyme. But they turned out to be toxic not only to trypanosomes, but also to the human body [5].
Fortunately, despite all the twists and turns of fate, scientists have once again focused their attention on irreversible covalent inhibitors. A better understanding of the actual risks and knowledge of the mechanism of chemical transformations has opened up many opportunities for the development of more effective drugs and has led to a sharp increase in the occurrence of irreversible inhibitors in various studies [10].