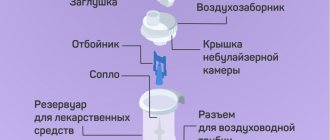
Diseases caused by respiratory infections can last for weeks if sputum is difficult to clear from the walls of the bronchi and lungs. And tracheitis turns into endless irritation of the throat, accompanied by a cough that tears at the walls. Acetylcysteine helps phlegm to separate from the walls of the respiratory system, accelerating the healing process.
Acetylcysteine: what is it?
Acetylcysteine is a substance actively used for diseases of the respiratory system. It can be purchased both in powder form for preparing a solution and in tablets. Tablets can be soluble and effervescent, or you can find a form for swallowing with water. Acetylcysteine is found under different trade names.
The use of the medicine reduces the viscosity of mucus produced by the respiratory system against the background of the inflammatory process. Due to this, the mucus becomes liquid and easily moves away from the walls of the bronchi and bronchioles. In addition, the product increases the volume of phlegm. If the patient is bedridden or suffers from cough reflex disorders, medical practice uses a special suction system that protects the lungs from filling with mucus.
Additionally, Acetylcysteine reduces the severity of the inflammatory process by suppressing reactive metabolites in the lung tissue, thereby inhibiting the development of the acute course of the disease.
Oral administration ensures complete and rapid absorption of the substance into the blood, which contributes to the rapid onset of the first symptoms of relief. Most of Acetylcysteine is excreted from the body by the kidneys. A small part comes out unchanged during bowel movements. The maximum concentration is reached after 2.5 hours. Withdrawal may be delayed in patients with liver damage, causing the drug to remain in the body for up to 8 hours.
ANTIOXIDANT AND ANTI-TOXIC PROPERTIES OF ACETYL CYSTEINE
ANTIOXIDANT PROPERTIES OF ACETYL CYSTEINE
There are a number of medicines, interest in which not only does not wane over time, but also intensifies, opening up new areas and possibilities for their use. Among the drugs whose potential has not yet been exhausted is, of course, acetylcysteine (N-acetylcysteine, NAC, ACC). Over the past 2–3 years alone, the world literature has published the results of hundreds of studies in various fields of medicine devoted to new areas of use of acetylcysteine or the expansion of existing indications. Acetylcysteine is a derivative of the amino acid cysteine and has a mucolytic and expectorant effect. Typically, the drug is used to dilute sputum in diseases of the respiratory system, accompanied by the formation of thick secretions.
Due to the presence of a free sulfhydryl group in the molecule, acetylcysteine breaks the disulfide bonds of acidic sputum mucopolysaccharides, which reduces their polymerization and the viscosity of bronchial secretions, and accelerates mucociliary clearance.
In addition, due to the presence of a free sulfhydryl group, acetylcysteine has a pronounced antioxidant, antitoxic, immunomodulatory effect, neutralizes free radicals formed in the body even during normal metabolic activity. Most researchers believe that free radicals, which have an adverse effect on cells and DNA, are the most important factor involved in the process of biological aging of the body. Thanks to its pronounced antioxidant and pneumoprotective properties, acetylcysteine provides protection for the respiratory system from the toxic effects of adverse environmental factors, such as tobacco smoke.
Acetylcysteine activates the synthesis of glutathione, an important factor in chemical detoxification (Anderson ME, Luo JL, 1996).
Acetylcysteine protects the body's cells from the influence of free radicals both by directly reacting with them and by supplying cysteine for the synthesis of glutathione. A number of researchers believe that it is the second mechanism of action of the drug that is most important (Shattuck K.E. et al., 1998). The level of glutathione in the body's cells largely determines resistance to oxidative influences and tends to decrease with age.
In addition, the drug can be used as an immunomodulator and antimutagenic agent (Ostrumova M.N. et al., 1994), as well as a radioprotector in persons exposed to ionizing radiation.
ACETYL CYSTEINE AS AN ANTIOXIDANT
In 1989, OT Aruoma et al., in an in vivo
and
in vitro
antioxidant properties of acetylcysteine revealed its
nonspecific activity in neutralizing various free radical groups.
Acetylcysteine exhibits a pronounced antioxidant effect even in low concentrations.
Obviously, this happens not only at the biochemical, but also at the biological level.
Acetylcysteine protects cells, in particular oligodendrocytes and fibroblasts, from death due to the influence of various unfavorable factors - programmed cell death (apoptosis) caused by exposure to an inadequate amount of tumor necrosis factor (TNF). This conclusion was reached by M. Mayer and M. Noble (1994), studying in vitro
properties of acetylcysteine as a cellular protector.
These data are comparable with the results presented by the team of authors regarding apoptosis of vascular endothelial cells. According to one of the existing pathophysiological models, exposure to peripheral blood mononuclear cells activated by ionizing radiation or bacterial endotoxins (lipopolysaccharides, LPS) blocks the restoration of damaged vascular endothelium and increases apoptosis. This mechanism may underlie various clinical complications involving the internal vascular wall.
The experiment established that taking acetylcysteine completely prevented the induced programmed death of vascular endothelial cells.
These results open up prospects for the use of acetylcysteine in pathological processes that have an adverse effect on the inner wall of blood vessels (for example, toxemia of any origin) (Lindner H. et al., 1997).
The cytoprotective effect of acetylcysteine is closely related to its antioxidant properties and determines the areas of possible use of the drug. Naturally, clinicians around the world are primarily interested in the potential effect of acetylcysteine in various diseases and pathological conditions.
According to K. Erkkila et al (1998), antioxidant defense plays an important role in the regulation of programmed cell death, even if this death is induced by non-oxidative stimuli. The results of this study indicate the role of acetylcysteine as an inhibitory factor in vitro
programmed death of germinal epithelial cells of the seminiferous tubules.
The authors consider acetylcysteine as a potentially effective treatment for idiopathic oligospermia.
The article by S. Fiorentini et al (1999) provides evidence of the effectiveness of the use of acetylcysteine for the prevention of pseudomembranous colitis caused by the use of antibiotics.
The authors indicate that the inducing factor in the development of this pathology is toxins A and B of the anaerobic bacterium Clostridium difficile.
At the subcellular level, these toxins have a damaging effect on the actin cytoskeleton. The results of a study of the effect of disturbances in the redox balance on the toxin-induced cytopotic effect indicate that both toxins provoke oxidative stress, as a result of which the number of protein SH groups significantly decreases.
We present the first evidence that the thiol group supplier acetylcysteine prevents cellular damage by acting on cytoskeletal integrity.
Currently, the main area of application of acetylcysteine is pulmonology.
A few years ago, the antioxidant properties of acetylcysteine were recognized as an important factor that has an additional impact on the course of the pathological process in the lungs. The fact is that inflammatory lung diseases are characterized by a sharp increase in oxidative processes in the lung tissue. This leads to a decrease in glutathione content, which in turn is accompanied by impaired surfactant function and increased activity of cytokinins, mediators of inflammation. Normally, intracellular glutathione suppresses the production of inflammatory mediators and prevents direct damage to lung tissue under the influence of free radical reactions. The supplier of thiol groups, acetylcysteine, helps restore intracellular glutathione content and has a direct antioxidant effect. This creates additional benefits when using acetylcysteine in inflammatory lung diseases (
Morris PE, Bernard GR, 1994).
Another important advantage of using acetylcysteine was noted by S.N. Zheng et al (1999). The influence of the mucoregulatory agents carboxymethylcysteine and acetylcysteine on the adhesive properties of the bacterium Morahella
catarrhalis
in relation to pharyngeal epithelial cells was studied.
When using these drugs, the adhesive properties of M. cafarrhalis
decreased in a dose-dependent manner - by a maximum of 57% of the initial value. These results allow us to conclude that an additional factor influencing the effectiveness of acetylcysteine treatment in patients with acute and chronic respiratory diseases is the ability of the drug to inhibit the adhesion of bacteria to the epithelium of the upper respiratory tract.
In 1995, the results of a double-blind study of the effectiveness of acetylcysteine in 24 patients operated on using a cardiopulmonary bypass (CAB) were published. Of these, 12 patients received acetylcysteine as a bolus at a dose of 100 mg/kg against the background of continuous drip administration of acetylcysteine at a dose of 20 mg/kg per hour (group 1); 12 - placebo (2nd, control group).
To determine the oxidative reaction of neutrophil granulocytes (NG), blood was taken several times at different time intervals. In patients of group 1, the oxidative reaction of NG was significantly less pronounced than in patients in the control group.
Based on these data, the authors determine the role of acetylcysteine as a neutralizer of oxygen free radicals, effective in
performing
operations using AIK
(Andersen LW et al., 1995).
The results of another prospective randomized study were recently published (Porter JM et al., 1999). The important role of oxygen radicals in the etiology of multiple organ failure syndrome and infectious complications through direct cellular toxicity and activation of intracellular mediator processes has been established.
A decrease in the activity of antioxidant defense was revealed in patients with traumatic injuries. There was a decrease in the content of glutathione, for which acetylcysteine is a precursor, and selenium, which is a cofactor for glutathione.
Eighteen patients with various injuries were divided into two groups. Patients of one of them were prescribed acetylcysteine, selenium, vitamins C and E for 7 days.
Compared with the control group, in the group of patients receiving active therapy, infectious complications (8 versus 18) and organ dysfunction occurred less frequently (0 versus 9). These data support the view that the use of antioxidant agents, particularly acetylcysteine, helps reduce the incidence of infectious complications and organ dysfunction
in patients with serious trauma.
Additional data confirming the effectiveness of acetylcysteine in various disorders of internal organ function (in this case, hepatorenal syndrome
), given by S. Holt et al (1999).
The authors note that the use of acetylcysteine improves kidney function
.
RW Hurd et al (1996) in 4 patients with progressive myoclonic epilepsy of the Unverricht-Lundborg type (PME-UL)
found increased activity of the extracellular enzyme superoxide dismutase.
This served as the basis for including acetylcysteine in the treatment plan at a daily dose of 4000–6000
mg.
Under the influence of treatment, a significant decrease in myoclonus and normalization of somatosensory-induced potentials were noted. Patients were treated with acetylcysteine for 30 months
.
A positive effect was also noted for a long time after stopping the drug. The authors concluded that acetylcysteine may prevent clinical deterioration in patients with PME-UL. In addition, the results presented can be considered as evidence of the safety of acetylcysteine
, since its use in doses many times higher than the average therapeutic, and for a time many times longer than the average duration of the usual course of treatment, did not lead to the development of serious side effects. The authors suggested that acetylcysteine can be used to improve the condition of patients with other neurodegenerative processes in which progression is caused by excess production of free radicals.
A similar hypothesis was expressed by M. Matinez et al. (1999). They proposed the use of acetylcysteine as a treatment for patients with Parkinson's disease.
substantia nigra
cells in Parkinson's disease, which is accompanied by a decrease in ATP synthesis and the accumulation of reactive oxygen radicals, the authors suggested that it is the excess of reactive radicals that leads to the death of
substantia nigra
and the appearance of clinical signs of the disease.
According to the authors, if the hypothesis is correct, then antioxidants containing a thiol group as a necessary component of oxidative phosphorylation will be able to protect cellular structures from oxidative damage. It is assumed that the use of antioxidants containing a thiol group may become a new strategy in the neuroprotective therapy of Parkinson's disease.
In most countries, antioxidant therapy is included in the standard treatment protocol for burn patients. A decrease in cellular immunity plays an important role in the pathogenesis of this type of damage.
O. Cetinkale et al (1999) published the results of a study conducted on rats to study the effect of antioxidant therapy on the state of cellular immunity after burn injury. Various antioxidant drugs were used: allopurinol (50 mg/kg per day), deferoxamine (15 mg/kg per day), PEG-catalase (PEG-CAT) (1200 units/kg per day), ascorbic acid (0.5 mg/kg per day). kg per day), acetylcysteine (1 mg/kg per day). All drugs were prescribed immediately after the burn and were used for 7 days. The data obtained indicate that deep immunosuppression in severe burns can be largely prevented by timely administration of antioxidant therapy, in particular acetylcysteine.
In addition, the possibility of restoring cellular immunity due to such therapy was noted.
However, back in 1993, data appeared in the world medical literature about the complex effect of acetylcysteine on immunity in HIV-infected patients (Eylar E. et al., 1993).
The article provides evidence of the immunostimulating effect of acetylcysteine on T lymphocytes, in particular on mitogenesis, the production of interleukin-2 (IL-2) and the growth of T lymphocytes in cell culture. Referring to the work of other authors and data from our own in vitro studies,
evidence of the inhibitory effect of acetylcysteine on HIV replication, the authors recommend acetylcysteine for the treatment of HIV-infected patients.
According to M. Roederer et al (1993), in patients with AIDS, the severity of infectious complications has been established to depend on the degree of decrease in glutathione levels. When glutathione levels are low, the body's cells are subject to additional stress in the form of oxidative stress, which potentiates the development of infectious complications.
The authors recommend introducing acetylcysteine into the treatment regimen for patients with HIV infection as a means of inhibiting the development of any inflammatory processes, including those directly related to HIV replication. The same opinion is shared by W. Droge (1993).
The results of a number of clinical and laboratory studies suggest that the development of AIDS may be largely due to HIV-induced cysteine deficiency.
The supply of cysteine to the cell affects the intracellular content of glutathione, IL-2-dependent proliferation of T lymphocytes, and activation of the transcription factor NF-kappaB. Therefore, cysteine deficiency in HIV-infected patients is responsible not only for cellular dysfunction, but also for a number of pathogenetic factors of the disease.
The authors concluded that acetylcysteine can be considered as a means of replenishing cysteine and restoring glutathione levels in the body in HIV-infected patients. The expected effect is due to the fact that acetylcysteine affects various factors in the pathogenesis of AIDS and is a well-studied and safe drug. However, the effect of acetylcysteine in AIDS and other diseases is associated not only with its role as a supplier of cysteine for the synthesis of glutathione.
R. Kinscherf et al (1994) found in healthy individuals that with an excess level of intracellular glutathione, as well as with its deficiency, the number of CD4+ T cells is lower than with glutathione levels within the normal range. In patients with reduced intracellular glutathione levels, a decrease in the number of CD4+ T cells by an average of 30% was observed over 4 weeks. This work revealed that the purpose
acetylcysteine
prevented a decrease in the number of CD4+ T cells despite changes in intracellular glutathione levels.
Acetylcysteine has not only a direct, but also an indirect effect on the immune system. In one of the studies, for example, its inducing effect was noted in the treatment of patients with viral hepatitis C with interferon (Beloqui O. et al., 1993). Alpha interferon is an effective drug used to treat patients with hepatitis C, but resistance to the drug often develops, the mechanism of which is still unknown. In this study, with resistance to alpha interferon, that is, with a high level of alanine aminotransferase (ALAT) activity in the blood, patients were additionally prescribed acetylcysteine orally at a dose of 600 mg per day for 4 months of therapy. In all patients, before the use of acetylcysteine, a decrease in the content of glutathione in the blood plasma and in mononuclear cells was also noted. The effectiveness of further treatment was assessed based on the level of ALT in the blood plasma. When acetylcysteine was used in combination with alpha-interferon, ALT activity decreased; in 41% of patients, after 5–6 months it corresponded to normal values. Intracellular glutathione levels also recovered.
The latter fact partly explains the effectiveness of combination therapy for hepatitis C.
A 1998 study using feline immunodeficiency virus found that restoring intracellular glutathione levels had a direct inhibitory effect on viral replication. Acetylcysteine was also used as a means to normalize the level of intracellular glutathione (Mortola E. et al., 1998).
During exacerbation of intermittent porphyria, 5-aminolevulinic acid (5-ALA), a heme precursor, accumulates in the human body. This acid stimulates the production of free radicals, which leads to damage to proteins and DNA.
Obtained in vitro
Evidence suggests that acetylcysteine prevents free radical damage to proteins and DNA caused by the accumulation of 5-ALA (Yusof M. et al., 1999).
And another potential area of application for acetylcysteine. Data from recent experimental studies in mice have suggested the feasibility of using acetylcysteine in cytokine-mediated diseases, in particular arthritis (
Tsuji F. et al., 1999).
ANTI-TOXIC PROPERTIES OF ACETYL CYSTEINE
Acetylcysteine has pronounced nonspecific antitoxic activity
.
Its use is equally effective in “home therapy”, for example, to eliminate a hangover due to excessive alcohol consumption, and in the treatment of serious, life-threatening poisoning. Acetylcysteine combines the properties of both a nonspecific toxicotropic
antidote (entering into physical and chemical interactions with toxic substances in the human body) and
a toxicokinetic
antidote (affecting the rate of degradation processes of toxic molecules).
Acetylcysteine activates the synthesis of glutathione
, which is an important factor in chemical detoxification (Anderson ME, Luo JL, 1996).
Since the beginning of clinical use of the drug, information has been constantly accumulating on its effectiveness in case of poisoning by a wide variety of substances.
Acetylcysteine can be used in patients with acute poisoning by aldehydes, phenols and other chemicals.
Acetylcysteine had an antitoxic effect against a wide range of various toxic substances, in particular acrolein contained in cigarette smoke, car exhaust and fried foods, as well as cyclophosphamide formed in the body during the treatment of patients with cancer.
Acetylcysteine is an antidote for acute paracetamol (acetaminophen) poisoning.
Acetylcysteine is considered in the world medical literature as one of the most widely used antitoxic agents (Lheureux R. et al., 1990).
The literature also describes rare clinical cases of poisoning with a potentially fatal outcome, in which only the use of acetylcysteine could radically change the situation and save the patient.
In particular, the article by D. Mattin et al (1990) describes the case history of a 32-year-old patient who, 5.5 hours before admission to the intensive care unit, took a potentially lethal dose of one of the arsenic salts for suicidal purposes. Intramuscular administration of dimercaprol and additional detoxification measures were ineffective. The patient's condition worsened. After 27 hours, acetylcysteine was administered intravenously. Over the next 24 hours, the patient's condition improved significantly. A few days later he was discharged in satisfactory condition.
One of the reports from the California Center for Disease Control describes a case of recovery of a patient at the age of 42 years after poisoning with toadstool. Acetylcysteine in this case was prescribed orally in high doses. A few days later the patient was discharged in satisfactory condition. No long-term effects of poisoning were observed (Amanita phalloides Mushroom Poisoning, 1997).
Domestic authors have obtained data on the effective use of acetylcysteine in combination therapy of patients with acute dichloroethane poisoning. Acetylcysteine was used after extracorporeal detoxification (hemosorption) to prevent the development of toxic liver damage (Kurashov O.V., Trotsevich V.A., 1992).
In experimental work using the LLC-PK1 cell line in vitro
The effect of acetylcysteine on the cytotoxicity of cadmium was studied. It has been established that acetylcysteine effectively protects cells from the toxic effects of cadmium, mainly by reducing its penetration into the intracellular space (Wispriyono B. et al., 1998).
The effect of acetylcysteine on chronic cadmium-induced nephrotoxicity was studied.
Chronic cadmium-induced nephrotoxicity is characterized by irreversible damage to the kidneys in the late stages, and currently there are no methods for its elimination. The study was carried out on female Sprague–Dawley
who received 5 times a week for 26 weeks an injection of cadmium chloride at a dose of 5 µmol per 1 kg of body weight with additional use of acetylcysteine at the 13th week. The phenomena of nephrotoxicity (the appearance of protein and increased levels of lactate dehydrogenase in the urine) were noted at the 10th week.
The introduction of acetylcysteine, starting from the 13th week, prevented the further development of nephrotoxicity. Continuation of the administration of acetylcysteine after the 26th week (that is, after the cessation of cadmium administration) contributed to the rapid relief of symptoms even with severe nephrotoxicity (Shaikh ZA et al., 1999).
Sprague–Dawley rats
It has also been found that acetylcysteine significantly reduces
the nephrotoxicity of cyclosporine
associated with deterioration of blood supply to the kidneys and the accumulation of oxygen-derived free radicals (ODFR) in the kidney tissue.
For 3 weeks, animals of one group received cyclosporine at a dose of 25 and 50 mg/kg, the other group received cyclosporine in combination with acetylcysteine at various doses: 10, 20 and 40 mg/kg.
The effect was assessed by changes in the level of urea and creatinine in the blood plasma, as well as by histological examination of kidney biopsies. Treatment with acetylcysteine was very successful in protecting renal tissue from damage caused by cyclosporine. The role of oxidative stress in the pathogenesis of cyclosporine-induced nephrotoxicity has also been confirmed (Tiriq M. et al., 1999).
How common is mercury poisoning? A large number of people around the world are concerned about the increasing incidence of mercury poisoning due to the use of mercury-containing amalgams in dentistry.
This poisoning is called “amalgam-dependent non-allergic disease.” This term has not received the status of an official diagnosis, but is widely used in the literature when discussing the toxic effects of mercury.
The seriousness of this problem is evidenced, for example, by the fact that in Sweden there is an Association of Patients in whose treatment mercury amalgams were used. The association has existed for 16 years and has more than 15,000 members.
Of course, under normal conditions, the presence of mercury amalgam in the oral cavity does not create problems for a person. The problem will no longer seem far-fetched if you get acquainted with the research data of Sallsten (1996). The author found that in people who use nicotine-containing chewing gum, the mercury content in the urine increases 5 times and reaches the level formed upon contact with mercury under industrial conditions. Regular chewing gum has a similar effect, although to a lesser extent (Gay, 1979; Abraham, 1984; Aronsson, 1989; Berglund, 1990; Bjorkman, 1992).
Molin (1990) provides evidence that during dental procedures on a tooth with installed mercury amalgam, the mercury content in the blood plasma can increase by 300–400% compared to that before the manipulation.
In this regard, the property of acetylcysteine that reduces the toxic effect of mercury is of particular interest.
In a study conducted by F. Livardjani et al (1991), rats were exposed to mercury vapor. Histological examination revealed changes in the lung tissue - alveolar edema, the appearance of hyaline membranes and areas of fibrosis. Exposure to mercury vapor for 2 hours resulted in the death of 50% of animals over the next 2 weeks. The use of acetylcysteine increased life expectancy and the percentage of surviving animals, reduced the mercury content in the blood and lung tissue, and the activity of pulmonary superoxide dismutase (antioxidant effect).
Also, one study revealed the protective properties of acetylcysteine against nephrotoxicity caused by mercuric chloride. It was also noted that the use of acetylcysteine significantly reduces the mercury content in the tissues of the liver and kidneys (Girardi G., Elias MM, 1991).
In addition, the chelate properties of acetylcysteine were discovered during hemodialysis in patients with mercury poisoning. Its effectiveness against mercury was the highest compared to the other ten chelating agents commonly used for hemodialysis. 90 minutes after hemodialysis, the mercury content in the perfusate decreased by 73% (Ferguson CL, Cantilena LRJr., 1992).
Recently, data have been published showing that the use of acetylcysteine reduces the effect of arecoline, a carcinogen (Chatterjee A., Deb S., 1999).
"Ecstasy" is a slang name for 3,4-methylenedioxy-methamphetamine (MDMA); other slang names for this compound are “X”, “E”, “XTS”, “Adam”, etc. This semi-synthetic narcotic compound, usually in the form of white crystals of hydrochloric acid salt, causes mental dependence. Sold illegally in capsules, compressed powder, tablets or as a white powder. It is usually taken orally, but sometimes also intranasally, inhaled (smoked), administered subcutaneously, intramuscularly or intravenously.
Some researchers suggest that Ecstasy's effects on the serotonin system can be avoided or reduced by taking antioxidants.
B. Leibovitz (1999) suggests using 5 mg beta-carotene, 2 g bioflavonoids, 100 mg coenzyme Q, 2–4 g ascorbic acid, 1 g L-carnitine, 2 g N-acetylcysteine, 250 μg selenium, as a preventative measure. 1000 IU tocopherol.
These data are interesting, although they have not been properly confirmed.
ACETYL CYSTEINE AS AN ANTIDOTE IN PARACETAMOL POISONING
The use of paracetamol, an antipyretic and analgesic, is safe in recommended doses. This drug is one of the most widely used in the world. However, in high doses, paracetamol can cause severe liver damage. Paracetamol poisoning deserves a separate study, primarily because children are primarily victims of such poisoning. In the UK alone, 150 deaths due to paracetamol poisoning are recorded annually. Paracetamol, according to studies by different authors conducted at different times, was the cause of 5–45% of all household poisonings in the USA and Great Britain (Acute Exacerbations of COPD, 1998).
The literature describes cases of fulminant liver damage under the influence of the drug. The hepatotoxicity of paracetamol is associated with the accumulation of its toxic metabolite N-acetylimidoquinone. There is a high risk of developing liver necrosis when using the drug at a dose of 150 mg/kg in children and 10 g in adults. Acute forms of liver failure occur in 1–5% of cases of paracetamol overdose after 3–6 days from the start of use (Olson KR, 1994).
In patients with chronic alcoholism, hepatotoxicity may also occur during treatment with the drug in lower doses. These severe forms of liver damage occur in every second patient with alcoholism with severe liver dysfunction when taking paracetamol in therapeutic doses.
As the main antidote for paracetamol overdose
Currently,
acetylcysteine
intravenously or orally.
It was initially assumed that acetylcysteine
was effective only when administered no later than 14 hours after taking high-dose paracetamol.
However, experience in the practical use of the drug indicates that the outcome may be more favorable even if the antidote is administered after 24 hours.
However, a delay of more than 10 hours reduces the effectiveness of acetylcysteine (Olson KR, 1994).
In the United States, the practice is to use acetylcysteine only orally. In this case, the patient is first prescribed the drug in a loading dose of 140 mg/kg, then 17 doses of 10 mg/kg every 4 hours. This is a very effective methodological approach, but in practice its use is difficult, since paracetamol poisoning may be accompanied by vomiting that develops too late to facilitate the removal of paracetamol from the body. If vomiting follows immediately after taking the next dose of acetylcysteine, then taking the drug at this dose is immediately repeated (Olson KR, 1994).
There is another technique, according to which acetylcysteine is administered intravenously over 20 hours at a total dose of 300 mg/kg (Prescott LF et al., 1977).
Usually, with this administration, patients tolerate the drug well, but its effectiveness decreases if it is administered 10 hours or more after poisoning.
CONCLUSION
With a large number of drugs available, acetylcysteine is currently the drug of choice for non-specific detoxification and maintaining the proper level of antioxidant protection of the body.
Acetylcysteine is effective against poisoning with various organic and inorganic compounds. Acetylcysteine is well tolerated and absorbed, is resistant to enzymes and has proven activity when taken orally in numerous studies.
When is Acetylcysteine used?
The main indications for taking Acetylcysteine are bronchitis of any etiology and pneumonia, and it is also prescribed in cases of inflammation of the tracheal mucosa. The action of the drug is aimed at liquefying sputum, which is the result of the body’s local immunity.
When the pathogen enters the mucous membranes and provokes an inflammatory process, the lung tissue, as well as the epithelium lining the inner surface of the bronchi and bronchioles, begins to actively produce sputum. This is necessary in order to remove pathogenic microorganisms from the lumen of the respiratory system as soon as possible. Coughing in such a situation is another protective mechanism that removes infected mucus.
If the mucus is thick and viscous, it is difficult for it to separate from the walls of the respiratory organs. Settling in the bronchi and lungs, it becomes a constant irritant of cough receptors. In such a situation, a person suffers from a dry cough, and the disease progresses.
Acetylcysteine turns sputum into a liquid and non-sticky substance, which is completely eliminated in a couple of cough shocks. For pneumonia, Acetylcysteine helps the lungs get rid of mucus, in which pathogenic bacteria quickly multiply, causing the development of complications. By clearing the lungs of phlegm or preventing it from accumulating there, bacterial pneumonia can be avoided.
For sinusitis and sinusitis, Acetylcysteine is also often prescribed, since mucus in the upper respiratory tract can turn into otitis media. The sooner the mucus leaves the nasal passages and sinuses, the faster the recovery will occur.
Pharmacological use[edit | edit code]
Is mucolytic
, i.e. thins mucus, helping to remove it. Used for pulmonary diseases such as chronic obstructive pulmonary disease (COPD), bronchitis, etc.
Used for ARVI
. During viral infections, phagocytes are activated, resulting in increased production of free radicals and disruption of immune antiviral defense, which leads to cytotoxic effects that determine all the clinical symptoms of acute respiratory viral infection (ARVI).
In 1997, S. De Flora et al. conducted a multicenter placebo-controlled, double-blind, randomized study in which they showed the preventive effect of Fluimucil (acetylcysteine) in the development of ARVI. The drug did not directly affect antiviral immunity, but reduced the frequency of manifest forms of ARVI by 3 times and reduced the severity of both respiratory and general symptoms of the disease (headache, myalgia, arthralgia). The maximum effectiveness of the drug was observed during the period of the highest incidence of ARVI. When taking acetylcysteine, the disease was much easier, and recovery occurred faster.
Studies have shown that acetylcysteine enhances the effect of antibiotics.
Used for paracetamol poisoning and also as an antidote
. It has antitoxic properties and is used as an antidote for poisoning with paracetamol, as well as nitrates, car exhaust, cigarette smoke, heavy metals, etc. Protects against cigarette smoke.
The hepatotoxicity of paracetamol is associated with the accumulation of its toxic metabolite N-acetylamidoquinone, which depletes glutathione reserves in the liver. Acetylcysteine, by releasing intracellular L-cysteine, which takes part in the synthesis of glutathione, restores glutathione reserves and significantly improves the prognosis for paracetamol intoxication.
Acetylcysteine has a wide spectrum of antitoxic effects, including against acrolein, which is contained in cigarette smoke, car exhaust and fried foods, as well as formed in the body during treatment with cyclophosphamide. It is used as a chelating agent for metal poisoning, including mercury, gold, silver, cadmium, etc. Being a donor of free sulfhydryl groups, acetylcysteine has a protective effect against liver and kidney damage caused by mercury.
Experimental studies have demonstrated the ability of acetylcysteine to have a protective effect against the cytotoxic effects of cigarette smoke. Its prophylactic use suppressed smoking-induced hyperplasia of mucosal cells and epithelial hypertrophy. In addition, acetylcysteine helped improve microcirculation in smokers. The complex of favorable properties of the drug makes it possible to recommend its regular use not only for active, but also for passive smokers who are constantly exposed to cigarette smoke.
Restrictions on taking Acetylcysteine
Due to the fact that the drug is aimed at increasing the volume of sputum produced and diluting it, the drug is not taken at night. Otherwise, the night hours will be devoted not to sleep, but to productive coughing. This is especially important for children whose night cough will keep the whole family from sleeping.
Additionally, it should be noted that Acetylcysteine is not prescribed to children under two years of age. This is due to the fact that the child does not have a pronounced cough reflex or the force of his pushes is not strong enough. The drug can only be used in a hospital setting, when doctors, using special equipment, can suck out sputum from the child’s respiratory tract.
Use as an antioxidant[edit | edit code]
Acetylcysteine is a powerful antioxidant that has both direct and indirect effects. The direct effect is due to the presence of a thiol SH group capable of neutralizing free radicals, and acetylcysteine exhibits a pronounced antioxidant effect even at low concentrations. The indirect effect occurs due to the fact that acetylcysteine is a precursor to glutathione. Glutathione is a tripeptide that consists of glutamic acid, cysteine and glycine. Glutathione serves as a major protective factor against the effects of internal toxic and external agents: nitrogen oxide, sulfur oxide and other components of tobacco smoke, as well as air pollutants.
Possible adverse reactions
Rare adverse reactions include:
- headache;
- hives;
- swelling of facial tissues;
- decreased blood pressure;
- vomit;
- stomatitis.
At the first signs of negative reactions, you should immediately stop taking the drug. If the medicine was taken no more than half an hour ago, you should rinse the stomach, inducing vomiting.
Contraindications[edit | edit code]
Side effects when consuming acetylcysteine are extremely rare and are usually limited to gastrointestinal disorders. In case of overdose, diarrhea, nausea, and vomiting are possible.
The thiol group of acetylcysteine usually interacts with antibiotics, destroying them, so it is advisable to take antibiotics after 2 hours after taking acetylcysteine.
The thiol group of acetylcysteine can react with rubber and metals, so it is advisable to use glass or enamel dishes.
Dangerous interaction
Due to the fact that the mechanism of action of the drug is aimed at increasing the amount of liquid sputum in the respiratory organs, simultaneous use with drugs of reverse action is contraindicated.
Among them:
- Sinecode;
- Omnitus;
- Codelac Neo;
- Tussicode;
- Stoptussin.
All of them have butamirate as an active ingredient, which “switches off” the cough center in the brain, stopping the cough. As a result, sputum will be produced in huge quantities, filling the bronchi and lungs, unable to escape.
Additionally, other prescription cough suppressants, such as codeine-based Codelac, should also not be taken with mucolytics.
Combined use[edit | edit code]
- In case of illness.
As mentioned above, acetylcysteine enhances the effect of antibiotics, but only those that do not interact with the thiol group of acetylcysteine, for example thiamphenicol. In this case, the effect of acetylcysteine and the antibiotic is increased compared to using them separately.
- Antioxidant.
The work of Belarusian scientists (O.A. Borisenok) recommends combining melatonin and acetylcysteine. Melatonin, like acetylcysteine, is a strong antioxidant.