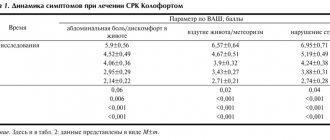
Metacin
— Diseases of the cardiovascular system, in which an increase in heart rate (HR) may be undesirable: atrial fibrillation, tachycardia, chronic heart failure and (CHF), coronary heart disease (CHD), mitral stenosis, arterial hypertension, acute bleeding;
- increased body temperature and febrile symptoms (the temperature may further increase due to suppression of the activity of the sweat glands);
- reflux esophagitis, hiatal hernia, combined with reflux esophagitis (decreased motility of the esophagus and stomach and relaxation of the lower esophageal sphincter can slow down gastric emptying and increase gastroesophageal reflux);
- diseases of the gastrointestinal tract (GIT), accompanied by obstruction: achalasia and pyloric stenosis (possibly decreased motility and tone, leading to obstruction and retention of gastric contents); intestinal atony in elderly or debilitated patients (possible development of obstruction), paralytic intestinal obstruction (possible development of obstruction);
- diseases with increased intraocular pressure: closed-angle (mydriatic effect, leading to an increase in intraocular pressure, can cause an acute attack) and open-angle glaucoma (mydriatic effect can cause a slight increase in intraocular pressure; therapy adjustment may be required), age over 40 years (risk of undiagnosed glaucoma);
- ulcerative colitis (high doses can inhibit intestinal motility, increasing the likelihood of paralytic ileus; in addition, the manifestation or exacerbation of such a severe complication as toxic megacolon is possible);
- dry mouth (long-term use may cause further increase in the severity of xerostomia);
- liver failure (decreased metabolism) and renal failure (risk of side effects due to decreased excretion);
- chronic lung diseases, especially in weakened patients (a decrease in bronchial secretion can lead to thickening of secretions and the formation of plugs in the bronchi);
- myasthenia gravis (the condition may worsen due to inhibition of the action of acetylcholine);
- autonomic (autonomic) neuropathy (urinary retention and paralysis of accommodation may increase), prostatic hyperplasia without urinary tract obstruction, urinary retention or predisposition to it, or diseases accompanied by urinary tract obstruction (including bladder neck due to prostatic hyperplasia );
- gestosis (possibly increased arterial hypertension);
- Down's disease (possibly unusual dilation of the pupils and increased heart rate);
- thyrotoxicosis and diseases of the thyroid gland (with disruption of its function);
- alcoholism.
Metacin
Metacin tablet 2 mg x10, ATX code: A03AB (Synthetic anticholinergics - quaternary ammonium compounds) Active substance: metocinium iodide (metocinium iodide) Rec.INN registered by WHO
Dosage form
Metacin
Tab. 2 mg: 10 pcs.reg. No.: LSR-008566/10 dated 08/23/10 - Valid
Release form, packaging and composition
Tablets 1 tab.
metocinium iodide 2 mg, Clinical and pharmacological group: M-cholinergic receptor blocker Pharmaco-therapeutic group: M- and n-cholinergic blocker
pharmachologic effect
M-cholinergic receptor blocker, monoquaternary compound. It has a predominant effect on peripheral m-cholinergic receptors. Reduces the tone of the smooth muscles of internal organs, reduces the secretion of gastrointestinal glands, bronchial and sweat glands, increases heart rate, improves AV conductivity. The ability to cause pupil dilation, increased intraocular pressure and paralysis of accommodation is much less pronounced than that of atropine.
Does not have a central effect.
Indications for the drug: Spasm of smooth muscles of internal organs, peptic ulcer of the stomach and duodenum, chronic gastritis, biliary and renal colic, hypersecretion of the salivary and bronchial glands, premedication before surgery to reduce the secretion of the salivary and bronchial glands, prevention of miscarriage, premature birth. ICD-10 codes
Dosage regimen The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed.
Individual. Adults orally - 2-4 mg 2-3 times a day. SC, IM or IV - 0.5 mcg-1 mg 2-3 times a day.
Maximum doses: single dose for oral administration - 5 mg, daily dose - 15 mg, single dose for parenteral administration - 2 mg, daily dose - 6 mg.
Side effects Possible: dry mouth, tachycardia, thirst, dysphagia, constipation, urinary retention, dilated pupils, paralysis of accommodation, increased intraocular pressure.
Contraindications for use: Hypersensitivity to methocinium iodide.
Use during pregnancy and breastfeeding Methocinium iodide is used to relieve increased excitability of the uterus when there is a threat of premature birth and late miscarriages, for premedication during cesarean section operations, because. it reduces the amplitude, duration and frequency of uterine contractions.
Use for liver dysfunction Use with caution in case of liver failure.
Use for impaired renal function Use with caution in case of renal failure (risk of side effects due to decreased excretion).
Use in children Use with caution in case of chronic lung diseases in young children (a decrease in bronchial secretion can lead to thickening of secretions and the formation of plugs in the bronchi), brain damage in children (the effects on the central nervous system may increase), Down's disease (possibly unusual expansion pupils and increased heart rate), central paralysis in children (the reaction to anticholinergic drugs may be most pronounced).
Use in elderly patients Caution should be used in cases of intestinal atony in elderly patients (possible development of obstruction), as well as in patients over 40 years of age (risk of undiagnosed glaucoma).
Special instructions Caution should be used for diseases of the cardiovascular system, in which an increase in heart rate may be undesirable - atrial fibrillation, tachycardia, chronic heart failure, coronary artery disease, mitral stenosis, arterial hypertension, acute bleeding, with thyrotoxicosis (possibly increased tachycardia), with elevated body temperature (may further increase due to suppression of the activity of the sweat glands), with reflux esophagitis, hiatal hernia combined with reflux esophagitis (decreased motility of the esophagus and stomach and relaxation of the lower esophageal sphincter can slow down gastric emptying and increase gastroesophageal reflux through sphincter with impaired function), in diseases of the gastrointestinal tract accompanied by obstruction - achalasia and pyloric stenosis (possibly decreased motility and tone, leading to obstruction and retention of gastric contents), intestinal atony in elderly or debilitated patients (possible development of obstruction), paralytic obstruction intestines (possible development of obstruction), in diseases with increased intraocular pressure - closed-angle (mydriasis, leading to increased intraocular pressure, can cause an acute attack) and open-angle glaucoma (mydriatic effect can cause a slight increase in intraocular pressure, adjustment of therapy may be required), older age 40 years (risk of undiagnosed glaucoma), with nonspecific ulcerative colitis (high doses can inhibit intestinal motility, increasing the likelihood of paralytic ileus, in addition, the manifestation or exacerbation of such a severe complication as toxic megacolon is possible), with dry mouth (long-term use may cause a further increase in the severity of xerostomia), with liver failure (decreased metabolism) and renal failure (the risk of side effects due to decreased excretion), with chronic lung diseases, especially in young children and debilitated patients (a decrease in bronchial secretion can lead to thickening of secretions and the formation of plugs in the bronchi), myasthenia gravis (the condition may worsen due to inhibition of the action of acetylcholine), autonomic neuropathy (urinary retention and paralysis of accommodation may increase), prostatic hypertrophy without urinary tract obstruction, urinary retention or predisposition to it or diseases, accompanied by obstruction of the urinary tract (incl. bladder neck due to prostatic hypertrophy), with gestosis (possibly increased arterial hypertension), with brain damage in children (CNS effects may increase), Down's disease (possibly unusual dilation of the pupils and increased heart rate), central paralysis in children (reaction on anticholinergic drugs may be most pronounced), tachycardia (may increase).
Drug interactions When used simultaneously with m-anticholinergic blockers, drugs with anticholinergic activity, the anticholinergic effect may be enhanced. ,
Conditions for dispensing from pharmacies By prescription
Metacin tablet 2 mg pack cell cont x10
Metacin tablet 2 mg x10, ATX code: A03AB (Synthetic anticholinergics - quaternary ammonium compounds) Active substance: metocinium iodide (metocinium iodide) Rec.INN registered by WHO
Dosage form
Metacin
Tab. 2 mg: 10 pcs.reg. No.: LSR-008566/10 dated 08/23/10 - Valid
Release form, packaging and composition
Tablets 1 tab.
metocinium iodide 2 mg, Clinical and pharmacological group: M-cholinergic receptor blocker Pharmaco-therapeutic group: M- and n-cholinergic blocker
pharmachologic effect
M-cholinergic receptor blocker, monoquaternary compound. It has a predominant effect on peripheral m-cholinergic receptors. Reduces the tone of the smooth muscles of internal organs, reduces the secretion of gastrointestinal glands, bronchial and sweat glands, increases heart rate, improves AV conductivity. The ability to cause pupil dilation, increased intraocular pressure and paralysis of accommodation is much less pronounced than that of atropine.
Does not have a central effect.
Indications for the drug: Spasm of smooth muscles of internal organs, peptic ulcer of the stomach and duodenum, chronic gastritis, biliary and renal colic, hypersecretion of the salivary and bronchial glands, premedication before surgery to reduce the secretion of the salivary and bronchial glands, prevention of miscarriage, premature birth. ICD-10 codes
Dosage regimen The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed.
Individual. Adults orally - 2-4 mg 2-3 times a day. SC, IM or IV - 0.5 mcg-1 mg 2-3 times a day.
Maximum doses: single dose for oral administration - 5 mg, daily dose - 15 mg, single dose for parenteral administration - 2 mg, daily dose - 6 mg.
Side effects Possible: dry mouth, tachycardia, thirst, dysphagia, constipation, urinary retention, dilated pupils, paralysis of accommodation, increased intraocular pressure.
Contraindications for use: Hypersensitivity to methocinium iodide.
Use during pregnancy and breastfeeding Methocinium iodide is used to relieve increased excitability of the uterus when there is a threat of premature birth and late miscarriages, for premedication during cesarean section operations, because. it reduces the amplitude, duration and frequency of uterine contractions.
Use for liver dysfunction Use with caution in case of liver failure.
Use for impaired renal function Use with caution in case of renal failure (risk of side effects due to decreased excretion).
Use in children Use with caution in case of chronic lung diseases in young children (a decrease in bronchial secretion can lead to thickening of secretions and the formation of plugs in the bronchi), brain damage in children (the effects on the central nervous system may increase), Down's disease (possibly unusual expansion pupils and increased heart rate), central paralysis in children (the reaction to anticholinergic drugs may be most pronounced).
Use in elderly patients Caution should be used in cases of intestinal atony in elderly patients (possible development of obstruction), as well as in patients over 40 years of age (risk of undiagnosed glaucoma).
Special instructions Caution should be used for diseases of the cardiovascular system, in which an increase in heart rate may be undesirable - atrial fibrillation, tachycardia, chronic heart failure, coronary artery disease, mitral stenosis, arterial hypertension, acute bleeding, with thyrotoxicosis (possibly increased tachycardia), with elevated body temperature (may further increase due to suppression of the activity of the sweat glands), with reflux esophagitis, hiatal hernia combined with reflux esophagitis (decreased motility of the esophagus and stomach and relaxation of the lower esophageal sphincter can slow down gastric emptying and increase gastroesophageal reflux through sphincter with impaired function), in diseases of the gastrointestinal tract accompanied by obstruction - achalasia and pyloric stenosis (possibly decreased motility and tone, leading to obstruction and retention of gastric contents), intestinal atony in elderly or debilitated patients (possible development of obstruction), paralytic obstruction intestines (possible development of obstruction), in diseases with increased intraocular pressure - closed-angle (mydriasis, leading to increased intraocular pressure, can cause an acute attack) and open-angle glaucoma (mydriatic effect can cause a slight increase in intraocular pressure, adjustment of therapy may be required), older age 40 years (risk of undiagnosed glaucoma), with nonspecific ulcerative colitis (high doses can inhibit intestinal motility, increasing the likelihood of paralytic ileus, in addition, the manifestation or exacerbation of such a severe complication as toxic megacolon is possible), with dry mouth (long-term use may cause a further increase in the severity of xerostomia), with liver failure (decreased metabolism) and renal failure (the risk of side effects due to decreased excretion), with chronic lung diseases, especially in young children and debilitated patients (a decrease in bronchial secretion can lead to thickening of secretions and the formation of plugs in the bronchi), myasthenia gravis (the condition may worsen due to inhibition of the action of acetylcholine), autonomic neuropathy (urinary retention and paralysis of accommodation may increase), prostatic hypertrophy without urinary tract obstruction, urinary retention or predisposition to it or diseases, accompanied by obstruction of the urinary tract (incl. bladder neck due to prostatic hypertrophy), with gestosis (possibly increased arterial hypertension), with brain damage in children (CNS effects may increase), Down's disease (possibly unusual dilation of the pupils and increased heart rate), central paralysis in children (reaction on anticholinergic drugs may be most pronounced), tachycardia (may increase).
Drug interactions When used simultaneously with m-anticholinergic blockers, drugs with anticholinergic activity, the anticholinergic effect may be enhanced. ,
Conditions for dispensing from pharmacies By prescription