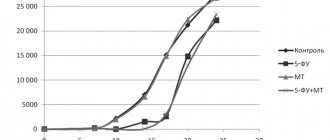
Human immunoglobulin is an immunological drug. It is a concentrated solution of an immunologically active protein fraction, which is isolated from the blood plasma of healthy donors by fractionation with ethyl alcohol at temperatures below 0°C.
To produce one batch of immunoglobulin, manufacturers use plasma obtained from at least 1000 healthy donors. They are preliminarily individually tested for the absence of surface antigen of the hepatitis B virus, antibodies to the hepatitis C virus and human immunodeficiency viruses.
At the Yusupov Hospital, immunoglobulins registered in the Russian Federation are used to treat patients. They are highly effective and have a minimal range of side effects.
Instructions for use
The active ingredients of normal human immunoglobulin are immunoglobulins, which contain antibodies of various specificities. The preparation contains from 9.5 to 10.5% protein. The maximum concentration of antibodies in the blood is determined 24-48 hours after administration of the drug. The half-life of antibodies is 4-5 weeks.
Normal human immunoglobulin (instructions are in the box) is available in the form of a solution in ampoules of 1.5 ml (1 dose). One package may contain 5, 10 or 20 ampoules of the drug. The kit includes an ampoule nail file. The drug is dispensed in pharmacies with a doctor's prescription. Immunoglobulin in ampoules is transported and stored at air temperatures from +2 to +8°C.
Immunoglobulin is injected intramuscularly into the outer upper quadrant of the buttock or the anterior surface of the thigh. The drug is not administered intravenously. Nurses at the Yusupov Hospital strictly follow the rules of asepsis and antisepsis when performing immunoglobulin injections. Before injection, ampoules with human immunoglobulin are kept for two hours at room temperature.
To prevent foam from forming in the syringe, the drug is drawn into the syringe with a wide-bore needle. It is injected by changing the needle. The drug in an opened ampoule cannot be stored. At the Yusupov Hospital, patients are not administered immunoglobulin if the integrity or labeling of the ampoules is damaged. The drug is unsuitable for use if the solution becomes cloudy, changes color, has flakes that do not break, as well as immunoglobulin that was stored in improper conditions or has expired.
Make an appointment
Use of the drug Human immunoglobulin
IV drip. The regimen of use is determined individually, taking into account the indications, severity of the disease, the state of the patient’s immune system and individual tolerance. The dosage regimens given below are advisory in nature. For primary immunodeficiency syndromes, a single dose is 200–800 mg/kg (average 400 mg/kg). Administered at intervals of 3–4 weeks to achieve and maintain a minimum level of IgG in the blood plasma of at least 5 g/l. For secondary immunodeficiency syndromes, a single dose is 200–400 mg/kg. Administer at intervals of 3–4 weeks. For the prevention of infections in patients undergoing bone marrow allotransplantation , the recommended dose is 500 mg/kg. It can be administered once 7 days before transplantation and then repeated once a week for the first 3 months after transplantation and once a month for the next 9 months. For idiopathic thrombocytopenic purpura, an initial single dose of 400 mg/kg is prescribed, administered for 5 consecutive days. It is possible to prescribe a total dose of 0.4–1 g/kg once or over 2 consecutive days. If necessary, further doses of 400 mg/kg can be administered at intervals of 1–4 weeks to maintain a sufficient platelet count. For Kawasaki syndrome, 0.6–2 g/kg is administered in several doses over 2–4 days. For bacterial infections (including sepsis) and viral infections, 0.4–1 g/kg is administered daily for 1–4 days. To prevent infection in premature infants with low birth weight, 0.5–1 g/kg is administered at intervals of 1–2 weeks. For Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy, 0.4 g/kg is administered for 5 consecutive days. If necessary, 5-day courses of treatment are repeated at intervals of 4 weeks. Depending on the specific situation, the lyophilized powder can be dissolved in 0.9% sodium chloride solution, water for injection or 5% glucose solution. The concentration of immunoglobulin in any of these solutions can vary from 3 to 12% depending on the volume used. For patients receiving immunoglobulin for the first time, it is recommended to administer it in the form of a 3% solution, and the initial infusion rate should be from 0.5 to 1 ml/min. If there are no side effects during the first 15 minutes, the infusion rate can be gradually increased to 2.5 ml/min. For patients who regularly receive and tolerate immunoglobulin, it can be administered in higher concentrations (up to 12%), but the initial infusion rate should be low. If there are no side effects, the infusion rate can be gradually increased.
Indications
Immunoglobulin is not used as a vaccine or medicine. It is designed to strengthen the body's defenses in the fight against diseases, as well as to develop a lasting barrier against them. Immunoglobulins contain antibodies that recognize harmful bacteria and viruses that enter the body, inhibit their reproduction and neutralize the toxic substances they produce.
Doctors at the Yusupov Hospital use normal human immunoglobulin to prevent various diseases:
- for immunodeficiency, immune diseases;
- in the postoperative period;
- for the prevention of diseases such as influenza, whooping cough, measles, meningococcal infection, polio, in addition, the drug has an antitetanus effect;
- for the prevention of tick-borne encephalitis;
- for various infectious diseases, including sepsis;
- for blood diseases;
- with acquired immunodeficiency syndrome (usually children), etc.
Doctors at the Yusupov Hospital prescribe immunoglobulin 25 ml for severe forms of bacterial-toxic and viral infections, sepsis, and dermatomyositis. The drug is included in the treatment regimen for Guillain-Barré syndrome, hyperimmunoglobulinemia E syndrome, Eaton-Lambert syndrome, and infections caused by parvovirus B9.
Neurologists prescribe simple human immunoglobulin to patients suffering from multiple sclerosis, chronic inflammatory demyelination in polyneuropathy, and myasthenia gravis. Pediatricians use the drug to prevent and treat infections in newborns, premature babies and children born with low birth weight.
After the introduction of normal human immunoglobulin, the overall resistance of the body increases during the recovery period of patients with infectious diseases.
Pharmacological properties of the drug Human immunoglobulin
Immunobiological agent, highly purified polyvalent human immunoglobulin. Immunoglobulin contains about 90% monomeric IgG and a small fraction of decomposition products, dimeric and polymeric IgG and IgA, IgM in trace concentrations. The distribution of IgG subclasses in it corresponds to their fractional distribution in human serum. It has a wide range of opsonizing and neutralizing antibodies against bacteria, viruses and other pathogens. In patients with primary or secondary immunodeficiency syndromes, it provides replenishment of missing IgG class antibodies, which reduces the risk of developing infection. In some other immune disorders, such as idiopathic (immune origin) thrombocytopenic purpura and Kawasaki syndrome, the mechanism of clinical effectiveness of immunoglobulin is not entirely clear. After IV infusion, a redistribution of immunoglobulin occurs between the blood plasma and the extravascular space, and equilibrium is achieved after approximately 7 days. Antibodies present in exogenous immunoglobulin have the same pharmacokinetic characteristics as antibodies in endogenous IgG. In individuals with normal serum IgG levels, the biological half-life of immunoglobulin averages 21 days, while in patients with primary hypogammaglobulinemia or agammaglobulinemia, the half-life of total IgG averages 32 days (however, there is significant individual variation that may be important when establishing a dosage regimen for a particular patient).
Contraindications
Normal human immunoglobulin should not be used by persons suffering from the following pathologies:
- hypersensitivity to human immunoglobulins;
- renal failure;
- diabetes mellitus;
- during exacerbation of allergic processes, etc.
Contraindications to the use of human immunoglobulin are severe allergic reactions to the administration of blood products in the past (allergic rashes, Quincke's edema, anaphylactic shock). The drug should not be used by patients suffering from systemic immunopathological diseases - connective tissue diseases, blood pathologies, nephritis. The use of simple immunoglobulin is contraindicated in thrombocytopenia and other disorders of the blood coagulation system.
Human immunoglobulin is used with caution in people with obesity, diabetes mellitus, and in the presence of risk factors for thrombotic complications (arterial hypertension, pathologies of the cardiovascular system, increased blood viscosity, old age). The drug is prescribed strictly under the supervision of a doctor in case of pathologies of the urinary system.
With extreme caution, normal human immunoglobulin is used for pregnant and lactating women, as well as for persons with decompensated chronic heart failure and suffering from migraines.
Side effects
Provided that all recommendations for dosage, administration and precautions are followed, serious side effects when using human normal immunoglobulin develop in extremely rare cases. Symptoms may appear several hours or even days after using the drug. As a rule, after stopping the use of immunoglobulin, side effects disappear.
Most often, side effects occur at a high rate of administration of the drug. By reducing the speed and temporarily stopping the intake, you can eliminate the majority of side effects. In other situations, symptomatic therapy is carried out.
The maximum likelihood of side effects occurs during the first use of the drug, during the first 60 minutes. Patients may experience the development of a flu-like syndrome with malaise, chills, fever, weakness, and headache.
In addition, the development of the following symptoms may be observed in various body systems:
- from the respiratory system: the appearance of a dry cough, shortness of breath;
- from the gastrointestinal tract: the appearance of nausea, diarrhea, vomiting, stomach pain, increased salivation;
- from the cardiovascular system: development of tachycardia, cyanosis, chest pain, flushing of the face;
- from the central nervous system (weakness, drowsiness, symptoms of aseptic meningitis (rarely) - nausea, vomiting, headache, photosensitivity, impaired consciousness, stiffness of the neck muscles);
- from the renal system: development of tubular necrosis, worsening renal failure in patients with renal dysfunction.
In addition to all of the above, when taking normal human immunoglobulin, the development of allergic reactions (skin rashes, bronchospasm, itching) and local reactions (hyperemia at the injection site) may occur.
It is extremely rare that when using the drug, loss of consciousness, collapse, or a sharp increase in blood pressure to significant levels may occur. In such situations, the drug must be discontinued and the patient must be immediately given antihistamines, adrenaline, and plasma replacement solutions.
After administration of human immunoglobulin, pain in the muscles of the limbs or back, numbness, fever, or a feeling of cold sometimes occurs. The skin at the injection site may turn red.
Make an appointment
HUMAN IMMUNOGLOBULIN AGAINST TICK-BORNE ENCEPHALITIS
Directions for use and doses
Injected intramuscularly, into the upper outer quadrant of the gluteal muscle or into the outer surface of the thigh. It is prohibited to administer the drug intravenously!
Before injection, the ampoule with the drug is kept for 2 hours at room temperature (20±2)°C. The opening of the ampoules and the administration procedure are carried out in strict compliance with the rules of asepsis and antiseptics. To avoid foam formation, the drug is drawn into a syringe with a wide bore needle.
The drug cannot be stored in an opened ampoule.
The dose of immunoglobulin and the frequency of its administration depend on the indications for use.
Prevention
Prevention before a tick bite
The drug can be used before possible contact with the tick-borne encephalitis virus - a tick bite in an endemic area (pre-exposure prophylaxis). The protective effect appears after 24-48 hours and lasts about 4 weeks. To maintain immunological protection in case of danger of infection, it is recommended to repeat the administration of immunoglobulin after 4 weeks.
For the purpose of prevention, the drug is administered intramuscularly once at the rate of 0.1 ml per 1 kg of body weight.
Table of prophylactic dosages of immunoglobulin against tick-borne encephalitis
| Body weight, kg | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Injection volume, ml | 0,5 | 1,0 | 2,0 | 3,0 | 4,0 | 5,0 | 6,0 | 7,0 | 8,0 |
Prevention after a tick bite
For the purpose of emergency prevention, the drug is administered primarily to those who have not been vaccinated against tick-borne encephalitis or who have received an incomplete course of vaccination, who have noticed tick bites in endemic areas, as well as in cases of suspected laboratory infection with the tick-borne encephalitis virus.
In cases of increased risk of infection (infection of a sucking tick, multiple bites or simultaneous sucking of several ticks is detected), the drug is also administered to vaccinated persons. Children under 12 years old - 1 ml; 12-16 years old - 2 ml; from 16 years and older - 3 ml.
In case of new contact with ticks, it is possible to re-use the drug one month after the first administration.
In all cases, the drug should be administered as early as possible from the moment of suspected infection, no later than 4 days after the tick bite.
Treatment
For therapeutic purposes, immunoglobulin is administered as early as possible after the onset of the disease in various dosages depending on body weight, clinical form of infection, severity and period of the disease.
— For patients with erased and abortive forms of tick-borne encephalitis (febrile forms of infection), immunoglobulin is administered daily in a single dose of 0.1 ml/kg body weight, for 3-5 days until the general infectious symptoms regress (improvement in general condition, disappearance of fever). The course average dose for an adult for these forms is at least 21.0 ml of the drug.
— For the meningeal form of tick-borne encephalitis, the drug is used daily in a single dose of 0.1 ml/kg body weight with an interval of 10-12 hours for at least 5 days until the patient’s general condition improves according to objective indicators (disappearance of fever, regression of general infectious symptoms, stabilization and reduction of meningeal symptoms). The course average dose of immunoglobulin for an adult is at least 70.0 ml for the meningeal form of infection.
— For patients with focal forms of tick-borne encephalitis, depending on the severity of the disease, the drug is administered daily in a single dose of 0.1 ml/kg body weight with an interval of 8-12 hours for at least 5-6 days until the temperature decreases and neurological symptoms stabilize. The average course dose for an adult patient is on average at least 80.0-130.0 ml of immunoglobulin.
In case of extremely severe disease, a single dose of the drug can be increased to 0.15 ml/kg body weight.
If patients with meningeal and focal forms of tick-borne encephalitis for some reason did not receive specific therapy during the febrile stage of the disease, it is possible to administer immunoglobulin at the apyrexia stage of the acute period of the disease for therapeutic purposes for 5-6 days in a single dose of 0.1 ml/kg body weight after 10-12 hours.
In the case of a two-wave course of tick-borne encephalitis, the drug is reused according to the treatment regimen for meningeal or focal forms, depending on the nature of the clinical manifestations.
Use of the drug during pregnancy
The role of immunoglobulins is especially important in the treatment of diseases in pregnant women. Their body is extremely sensitive to medications, many of them are simply contraindicated. Immunoglobulins are simply irreplaceable in this situation. They are non-toxic and do not harm the body. These are the drugs that increase the level of a person’s defense system and help him independently overcome the disease or prevent its development.
However, studies on the reaction of the body of pregnant women to the action of the drug have not been conducted, so there is no information about how harmful or harmless normal human immunoglobulin is during pregnancy and lactation. During pregnancy, the drug can be used in situations where there is an urgent need for its administration, if the potential benefits to the expectant mother's body outweigh the possible risks to the fetus.
During breastfeeding, normal human immunoglobulin should also be used with extreme caution, as it can enter the baby’s body through breast milk.
Drug interactions
Human normal immunoglobulin is considered pharmaceutically incompatible with other drugs. A separate dropper or syringe must be used for its administration. The simultaneous use of this drug with active immunization agents (for rubella, measles, chickenpox and mumps) may cause a decrease in the effectiveness of therapy. Live viral vaccines administered parenterally can be used no earlier than one month after the administration of normal human immunoglobulin.
In addition, in order to avoid the development of negative consequences, simultaneous administration of this drug with calcium gluconate to infants should not be allowed.
Drug interactions Human immunoglobulin
Concomitant use of immune globulin may reduce the effectiveness of active immunization against measles, rubella, mumps and varicella. In this regard, live viral vaccines for parenteral use should not be used for 6 weeks to 3 months after the use of immunoglobulin. In case of repeated administration in doses from 400 mg to 1 g/kg in children with idiopathic thrombocytopenic purpura or other pathology, vaccination against epidemic hepatitis should be postponed for 8 months. Immunoglobulin should not be mixed in the same volume with any other medications.
Conditions for dispensing from pharmacies
Normal human immunoglobulin (25 ml) is available by prescription. The decision to use normal human immunoglobulin should be made by a qualified specialist, based on the results of the examination and the characteristics of the patient’s body.
Independent use of the drug for other purposes may lead to negative consequences. You cannot buy immunoglobulin at non-specialized points of sale (from hand, according to an advertisement, with a large discount or a very low price), since in this case the storage conditions of the drug are often violated or the drug is not an immunoglobulin.
Special instructions for the use of human immunoglobulin
Due to the fact that in case of individual intolerance to the drug, patients may develop an immediate type of allergy, including anaphylactic shock, after the administration of simple human immunoglobulin, patients are under the supervision of a doctor at the Yusupov Hospital for 30 minutes. The drug is used with caution in case of impaired renal function. For children, pediatricians individually select the dose of the drug.
Since immunoglobulin reduces the effectiveness of vaccination, vaccinations are carried out no earlier than 2-3 months after administration of the drug. A temporary increase in antibodies in the blood after the administration of immunoglobulin leads to false-positive analysis data in a serological study. Pregnant women are administered simple human immunoglobulin only when the expected benefit to the mother outweighs the potential risk to the fetus. Immunoglobulin passes into breast milk and helps prevent infectious diseases in newborns.
It is recommended to observe the speed of administration, since faster administration may cause the development of collapse. The drug should be stored and transported at a temperature of +2 to +8°C. Immunoglobulin ampoules should not be frozen.
Normal human immunoglobulin - solution for infusion
special instructions
The drug is intended for single use.
After opening the bottle, the consumer is responsible for the duration of storage and storage conditions. Partially used drug cannot be stored or used. Unused drug and consumables should be disposed of in an appropriate manner.
The drug should not be used after the expiration date.
Patients should be closely monitored and monitored for any symptoms during the infusion period.
Persons who are injected with the drug must be under medical supervision for 30 minutes after its administration.
Infusion sites should be provided with anti-shock therapy.
The patient's condition should be closely monitored during drug infusion.
Some adverse reactions may occur more frequently:
- in case of high rate of administration; - in patients with hypogammaglobulinemia or agammaglobulinemia with or without IgA deficiency; - in patients who receive normal human immunoglobulin therapy for the first time, or in rare cases, when switching to another immunoglobulin drug, or after a long break after the previous infusion.
Possible complications can be avoided by making sure that:
- the patient does not exhibit hypersensitivity to normal human immunoglobulin with slow administration of the drug (0.5 mg/kg body weight/min); - During and after the infusion period, all symptoms experienced by patients are carefully monitored. In particular, patients who have not previously received human normal immunoglobulin therapy, who are switching from treatment with another intravenous immunoglobulin product, or who have had a long interval since the previous infusion, should be monitored during the first infusion and for the first hour after the first infusion to identify potential adverse events. All other patients should be observed for at least 30 minutes after administration of the drug.
If an adverse event occurs, the rate of administration should be reduced or the drug should be discontinued. The treatment required depends on the nature and severity of the adverse event.
If shock develops, standard treatment for shock conditions should be used.
All patients require adequate hydration before starting intravenous human immunoglobulin.
Hypersensitivity True hypersensitivity reactions are rare. They can occur in very rare cases with IgA deficiency with anti-IgA antibodies. Rarely, normal human immunoglobulin may cause a decrease in blood pressure with the development of an anaphylactoid reaction, even in patients who previously tolerated normal human immunoglobulin therapy well.
Hemolytic anemia Intravenous human immunoglobulin preparations may contain antibodies against blood group antigens, which can act as hemolysins and bind in vivo to red blood cells, which can cause a positive direct antiglobulin test (Coombs test) and, rarely, hemolysis. Hemolytic anemia may develop after therapy with intravenous human immunoglobulin preparations as a result of increased sequestration of red blood cells. Isolated cases of renal dysfunction and/or renal failure or disseminated intravascular coagulation syndrome associated with hemolysis have been reported.
The development of hemolysis is associated with the following risk factors:
high doses, whether administered as a single dose or in separate doses over several days; as well as blood groups A (II), B (III) and AB (IV) in combination with the concomitant presence of an inflammatory process. When treating patients with blood groups A (II), B (III) or AB (IV) with high doses of the drug for indications other than PID, extreme caution is recommended.
There are isolated reports of hemolysis in patients with PID receiving replacement therapy. Monitor clinical signs and symptoms of hemolysis in patients receiving intravenous human immunoglobulin therapy. If signs and/or symptoms of hemolysis occur during or after intravenous immunoglobulin infusions, the prescriber should consider discontinuing further treatment.
Aseptic meningitis syndrome (ASM) Cases of aseptic meningitis syndrome have been reported during treatment with intravenous immunoglobulin preparations. After discontinuation of intravenous immunoglobulin, MAS remission occurred within several days without any consequences. This syndrome usually begins within a few hours to 2 days after treatment with intravenous immunoglobulin. When analyzing cerebrospinal fluid, pleocytosis of up to several thousand cells per mm3 is often observed, usually due to cells of the granulocyte lineage, as well as an increased protein concentration, up to several hundred mg/dl. MAS may develop more frequently with the use of intravenous immunoglobulin in high doses (2 g/kg).
Thromboembolic complications There is clinical evidence of an association between the use of intravenous human immunoglobulin and the occurrence of thromboembolic complications, such as myocardial infarction, acute cerebrovascular accident (including stroke), pulmonary thromboembolism and deep vein thrombosis, which are presumably associated with a relative increase in viscosity blood with the introduction of a large amount of immunoglobulins. Caution must be exercised when prescribing and administering intravenous immunoglobulin infusions to obese patients and patients with previously established risk factors for thrombotic complications, such as advanced age, hypertension, diabetes mellitus, thromboembolism or a history of cardiovascular disease, hereditary or acquired thrombophilia, prolonged periods of impaired mobility, patients with severe hypovolemia and patients with diseases in which there is an increase in blood viscosity.
Acute renal failure Cases of acute renal failure have been identified in patients receiving intravenous human immunoglobulin therapy. In most cases, risk factors were identified, such as pre-existing renal failure, diabetes mellitus, hypovolemia, excess weight, concomitant treatment with nephrotoxic drugs, or age over 65 years. If renal failure develops, therapy with intravenous human immunoglobulin should be interrupted. It should be noted that among the reports of cases of renal dysfunction or renal failure developing while taking registered human immunoglobulin preparations, the proportion of preparations containing sucrose as a stabilizer was disproportionately high. Thus, for patients who are at risk, the use of intravenous immunoglobulin preparations that do not contain sucrose is recommended.
In patients at risk of developing acute renal failure or thromboembolic complications, intravenous immunoglobulin preparations should be administered at the minimum infusion rate and dose possible.
Effect on diagnostic tests After administration of immunoglobulins, the number of various passively transferred antibodies temporarily increases in the patient's blood, which can lead to a false-positive result in serological tests.
Passive transfer of antibodies to red blood cell antigens, such as A, B, and D, may cause an incorrect result in some serological tests for red blood cell antibodies (eg, Coombs test), reticulocyte count test, and haptoglobin test. Due to the presence of dextrose in the drug, it is possible to increase the concentration of glucose in the patient’s blood, which affects the result of its determination. Elevated blood glucose concentrations are determined during the period of drug administration and within 15 hours after drug administration. This fact must be taken into account when prescribing therapy for patients with diabetes.
Safety information regarding infectious agents The drug is produced from human plasma. Standard measures to prevent the transmission of infections resulting from the use of medicinal products made from human blood or plasma include selecting donors, screening individual donations and plasma pools for specific markers of infection, and incorporating effective manufacturing steps to inactivate and/or remove viruses . Despite this, when using drugs made from human blood or plasma, the possibility of transmission of infectious agents cannot be completely excluded. This provision also applies to unknown or new viruses and other infectious agents. Measures taken to ensure antiviral safety are considered effective for enveloped viruses such as HIV, hepatitis B and C viruses, and for non-enveloped viruses such as hepatitis A virus and parvovirus B19. There is encouraging clinical experience indicating that there is no transmission of hepatitis A virus and parvovirus B19 with human immunoglobulin preparations, and it is also suggested that the presence of antibodies makes a significant contribution to viral safety. It is recommended that each time a drug is used, the name and lot number of the drug administered to the patient is recorded to maintain a link between the patient and the drug lot. You should also record the date of release, expiration date, name of the manufacturer, date of administration and adverse reactions to the drug.
Impact on the ability to drive vehicles and machinery. Some adverse reactions associated with the action of the drug may affect the ability to drive a vehicle or operate machinery. For patients who have experienced adverse reactions during the administration of the drug, driving a vehicle or moving machinery is possible only after the symptoms of adverse reactions disappear.