Influenza vaccine (inactivated)
Used in children over 6 months, adolescents and adults. Manufacturer – Russia
Grippol® plus is an anti-influenza trivalent inactivated polymer-subunit vaccine. It is a protective antigen (hemagglutinin and neuraminidase) isolated from purified influenza viruses type A and B grown on chicken embryos, associated with a water-soluble high-molecular immunoadjuvant N-oxidized derivative of poly-1,4-ethylenepiperazine (azoximer bromide). The antigenic composition of the vaccine changes every year in accordance with the epidemic situation and WHO recommendations. The vaccine causes the formation of a high level of specific immunity against influenza. The protective effect after vaccination, as a rule, occurs after 8-12 days and lasts up to 12 months, incl. in elderly people. Protective titers of antibodies to influenza viruses after vaccination of people of different ages are determined in 75-95% of vaccinated people.
Influenza vaccine (inactivated)
Used in children over 6 months, adolescents and adults. Manufacturer – Russia.
Grippol® plus is an anti-influenza trivalent inactivated polymer-subunit vaccine. It is a protective antigen (hemagglutinin and neuraminidase) isolated from purified influenza viruses type A and B grown on chicken embryos, associated with a water-soluble high-molecular immunoadjuvant N-oxidized derivative of poly-1,4-ethylenepiperazine (azoximer bromide). The antigenic composition of the vaccine changes every year in accordance with the epidemic situation and WHO recommendations. The vaccine causes the formation of a high level of specific immunity against influenza. The protective effect after vaccination, as a rule, occurs after 8-12 days and lasts up to 12 months, incl. in elderly people. Protective titers of antibodies to influenza viruses after vaccination of people of different ages are determined in 75-95% of vaccinated people.
Grippol plus
The influenza vaccine “Grippol Plus” is a domestic vaccine, patented by the Institute of Immunology in 1995 and produced according to international GMP standards. Refers to the latest generation of inactivated polymer-subunit vaccines, which are characterized by safety and a low number of adverse reactions.
The vaccine contains antigens of 2 A-type viruses and one B-type antigen, as well as a substance that activates the body’s own immune response to fight viruses – polyoxidonium. The use of polyoxidonium allows the dose of antigens to be reduced threefold compared to other vaccines.
The Grippol Plus vaccine does not contain preservatives or antibiotics, internal virus antigens or toxic impurities in the form of lipids. The absence of preservatives is due to production conditions that comply with GMP standards, ensuring complete sterility through the use of an individual syringe dose.
The vaccine contains surface antigens of the virus, which ensure the appearance of antibodies on days 8-12, which last for almost a year.
Every year, the antigenic composition of Grippol Plus is updated in accordance with World Health Organization forecasts on the spread of the most dangerous strains of influenza viruses.
Purpose
Vaccination with Grippol Plus is recommended annually before the ARVI season.
The high safety and low reactogenicity of the vaccine allows us to recommend it for the prevention of influenza among various categories of citizens:
- children from 6 months, teenagers and adults;
- pregnant and lactating women;
- adults belonging to risk groups for severe influenza and ARVI;
- old people;
First of all, vaccination is necessary for people from risk groups who are in contact with a large number of people (medical workers, teachers, transport and service workers, students, military personnel), as well as people at high risk of developing severe forms of influenza and complications. People with heart diseases - coronary artery disease, hypertension, lung diseases - bronchial asthma, chronic bronchitis are exposed to this risk; diabetes mellitus, obesity.
Application
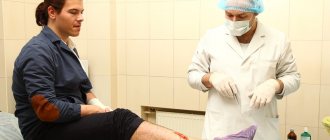
The flu vaccine "Grippol Plus" is available in the form of a ready-to-use injection suspension in an individual syringe dose and an atraumatic needle.
Intramuscular injection is carried out in compliance with the dosage and the period between administrations for different age groups - adults are recommended to administer one dose of the vaccine once, children from 6 months, depending on age and previous vaccination, may require 2 doses of the vaccine with an interval of 4 weeks.
The flu shot Grippol Plus can be administered simultaneously with other vaccines in the national schedule of preventive vaccinations. In this case, contraindications to each of the vaccines used must be taken into account.
Contraindications
Before vaccination, consultation with a doctor is required not only regarding the state of health before the Grippol plus vaccination, but also to identify possible contraindications:
- Individual intolerance to chicken egg whites, since the strains of viruses contained in the vaccine are grown on the proteins of chicken embryos;
- An allergic reaction (after a previous vaccination) to other components of the vaccine;
- Increased body temperature;
- The period of exacerbation of chronic diseases.
The price of the Grippol Plus vaccine at the Miracle Doctor clinic is similar to prices in other mid-price clinics. Before administering the vaccine, a consultation with a therapist or pediatrician is required, which is paid separately.
You can make an appointment for vaccination with our specialists by calling or visiting the clinic’s website.
pharmachologic effect
Grippol® plus is an anti-influenza trivalent inactivated polymer-subunit vaccine. The antigenic composition of the vaccine changes every year in accordance with the epidemic situation and WHO recommendations. The vaccine causes the formation of a high level of specific immunity against influenza. The protective effect after vaccination, as a rule, occurs after 8-12 days and lasts up to 12 months, incl. in elderly people. Protective titers of antibodies to influenza viruses after vaccination of people of different ages are determined in 75-95% of vaccinated people. The inclusion of the immunomodulator azoximer bromide in the vaccine preparation ensures an increase in the immunogenicity and stability of antigens, increases immunological memory, significantly reduces the vaccination dose of antigens, and increases the body's resistance to other infections by correcting the immune status.
Flu vaccination: difficulties of choice
Although vaccination against the influenza virus has already started, its relevance in November remains high. Those who want to protect themselves from a dangerous infection can still catch the last train if they administer the drug in the coming weeks. However, this year, adherents of influenza immunization have a problem of choice - imported Vaxigrip and Influvac have disappeared from access, and their place has been taken by domestic drugs, which some experts are skeptical about. Let's figure it out.
Production Features
First of all, it’s worth remembering once again what the flu vaccine is. The drug production process begins after the World Health Organization (WHO) publishes a list of strains of influenza viruses that will be distributed in the coming season. Armed with this information, manufacturers isolate three current strains of the H1N1, H3N2 and type B influenza viruses that circulate annually, inject them into a fertilized chicken egg, and grow the three-component preparation. It will contain surface proteins (antigens) of all three strains of the influenza virus - hemagglutinins. When they are administered, antibodies are formed in the body, which provide basic immunity against the virus.
Indications
Specific prevention of influenza in children from 6 months of age, adolescents and adults without age restrictions . The vaccine is especially indicated for people at high risk of complications from influenza:
- over 60 years old, preschool children, schoolchildren;
- adults and children who often suffer from acute respiratory infections, suffering from chronic somatic diseases, incl. diseases and malformations of the central nervous system, cardiovascular system and bronchopulmonary system, bronchial asthma, chronic kidney disease, diabetes mellitus, metabolic diseases, autoimmune diseases, allergic diseases (except allergy to chicken protein), chronic anemia, congenital or acquired immunodeficiency , HIV-infected;
The vaccine is also indicated for persons whose profession has a high risk of contracting influenza or infecting others with it, including:
- health workers, employees of educational institutions, social services, transport, trade, police, and military personnel.
Dosage regimen
Vaccination is carried out annually in the autumn-winter period.
- For children over 3 years of age, adolescents and adults, the vaccine is administered intramuscularly or deeply subcutaneously into the upper third of the outer surface of the shoulder (into the deltoid muscle), and for younger children - intramuscularly into the anterolateral surface of the thigh.
- Children aged 6 to 35 months inclusive are administered 0.25 ml twice with an interval of 4 weeks.
- For children over 36 months and adults, the vaccine is administered once in a dose of 0.5 ml.
- Children who have not previously had the flu and have not been vaccinated may receive the vaccine twice with an interval of 3-4 weeks.
Annual vaccinations against seasonal influenza significantly reduce morbidity and mortality in all age groups. The goal of vaccination is not to completely eliminate influenza as an infection, but to reduce morbidity and mortality from influenza and, especially, from its complications, from exacerbation and aggravation of cardiovascular, pulmonary diseases and other chronic pathologies.
Flu vaccines: what are they?
Every year, doctors recommend different drugs for vaccination. The fact is that influenza viruses change every season, and the composition of vaccines, accordingly, also needs to be changed.
There are two main types of influenza vaccines - live and inactivated. A live vaccine contains live viruses in a weakened state - they are called strains. The administration of live vaccines is often accompanied by side effects, so they are not currently included in prevention campaigns.
Inactivated vaccines do not contain live viruses. These drugs are characterized by high quality of purification - there are very few or practically no toxic impurities that are usually found in live vaccines. When inactivated influenza viruses are introduced into the body, the production of antibodies is activated, that is, exactly the same natural defense mechanisms are launched that are activated upon contact with living microorganisms.
We strongly advise you to consult a specialist who will recommend the vaccine most suitable for your body. No one can guarantee 100% that a vaccinated person will not get the flu, but it is 90% sure. And this is a serious argument to think about.
Vaccine "Sovigripp"
For active annual preventive immunization against seasonal influenza, the Sovigripp vaccine without a preservative is used in children from 6 months of age, adolescents and adults without any age limit, and in pregnant women in the II-III trimester of pregnancy; vaccine with 2 preservatives - in adults over 18 years of age.
“Sovigripp” is an effective and safe flu vaccine that has passed preclinical and clinical trials, confirming its high quality, which is not inferior to foreign analogues.
Benefits of the SOVIGRIPP vaccine
An effective and safe drug for the prevention of influenza, which has passed preclinical and clinical trials, confirming its high quality, which is not inferior to foreign analogues.
Sovigrippa contains the modern adjuvant Sovidon, which has immunomodulatory, detoxifying, antioxidant and membrane-protective properties.
Studies have shown that the drug has virtually no toxicity and pyrogenicity (increase in body temperature).
The ready-made dosage of Sovigrippa is as convenient as possible even for people without special medical knowledge.
Contraindications for use
1. Allergic reactions to chicken protein or other components of the vaccine.
2. Severe post-vaccination reactions (temperature above 40 °C, the appearance of edema at the site of vaccine administration, hyperemia over 8 cm in diameter) or post-vaccination complications (collapse, non-febrile convulsions, anaphylaxis) to the previous administration of influenza vaccine.
3. Pregnancy (when using a vaccine with a preservative).
4. Age up to 18 years (when using a vaccine with a preservative).
5. Age up to 6 months.
Temporary contraindications
1. Acute febrile conditions, acute infectious and non-infectious diseases, including the period of convalescence. Vaccination is usually carried out 2-4 weeks after recovery.
2. Chronic diseases in the acute stage. Vaccination is carried out during the period of remission. The possibility of vaccination of persons suffering from chronic diseases is determined by the attending physician, based on the patient’s condition.
3. For mild forms of acute respiratory viral and intestinal infections, vaccination is carried out after the temperature has normalized and/or the acute symptoms of the disease have disappeared.
Directions for use and doses
Vaccination is carried out annually in the autumn-winter period.
Vaccination is possible at the beginning of an epidemic rise in the incidence of influenza.
For children over 3 years of age, adolescents and adults without age restrictions, the vaccine is administered once intramuscularly into the upper third of the outer surface of the shoulder (in the area of the deltoid muscle) in a vaccination dose of 0.5 ml.
For children from 6 months to 3 years, the vaccine is administered twice with an interval of 4 weeks into the anterior outer surface of the thigh intramuscularly in a vaccination dose of 0.25 ml (1/2 dose) according to the administration schedule.
Precautions for use
- Do not administer intravenously!
- On the day of vaccination, those vaccinated must be examined by a therapist/paramedic with mandatory thermometry. At temperatures above 37 °C, vaccination is not carried out.
- Vaccination sites must be equipped with anti-shock therapy. The vaccinated person must be under medical supervision for 30 minutes after administration of the drug.
"Sovigrip." Questions and answers
Is it possible to vaccinate children with the Sovigripp vaccine? From what age?
Yes, you can. The Sovigripp vaccine is used for active annual preventive immunization against seasonal influenza in children from 6 months of age. In children from 6 months to 18 years of age and pregnant women, the vaccine is used without a preservative.
How do you know which vaccine is being administered, with or without a preservative?
All information about the Sovigripp vaccine is indicated on the packaging and ampoule. The childhood influenza vaccine is available only without a preservative.
Is it possible to get vaccinated with Sovigripp while taking antibiotics?
The vaccine can be administered against the background of basic therapy for the underlying disease. Vaccination in persons receiving immunosuppressive therapy may be less effective.
Is it possible to get vaccinated when planning a pregnancy? How long before expected pregnancy can I get vaccinated?
This issue should be discussed with your attending physician.
Can the vaccine have a negative effect on the gastrointestinal tract?
No. The vaccine does not have a negative effect on the organs and systems of the body. All possible reactions after vaccination are mainly transient in nature and resolve on their own without the need for additional therapy.
Can the Sovigripp vaccine with the Sovidon adjuvant cause allergies?
Analyzes carried out as part of clinical studies showed that vaccines with the Sovidon adjuvant do not have an allergenic effect.
I am afraid that the Sovigripp vaccine contains harmful components. Is it so?
There is a fear that has been raised by anti-vaxxers many years ago: vaccines contain components that are harmful to the human body. The scientific community has not ignored the situation of these fears and doubts. And WHO has conducted serious research into the health effects of vaccinated “dangerous” components. Tests have been carried out, with both short-term and long-term effects. Thousands of people were studied, but no negative health effects were found from vaccines. “Sovigripp” does not contain any components harmful to health; vaccination with it is safe and effective, which has been proven by clinical studies.
Is it possible to get the flu as a result of vaccination with Sovigripp?
No, It is Immpossible. Sovigripp, like other inactivated vaccines, contains only killed (and even split into components) influenza viruses. If the disease did occur after vaccination, then it can be explained either by the fact that at the time of vaccination the person was already in the incubation period of influenza, or by the fact that the person could have contracted another ARVI (the causes of which can be about 200 viruses). It is impossible to get the flu as a result of vaccination.
Is it possible to get vaccinated with Sovigripp during pregnancy?
Experience with the use of inactivated influenza vaccines shows that vaccination does not have a teratogenic or toxic effect on the fetus or child and can be used during pregnancy and breastfeeding. The final decision on vaccination of pregnant and breastfeeding women should be made individually by the doctor, taking into account the risk of contracting influenza and possible complications of influenza infection. Vaccination is safest in the second and third trimesters.
When is it more effective to vaccinate?
Experts recommend getting vaccinated at the beginning of the autumn-winter season. You can get the flu at any time, but the outbreak of infection in Russia is typical from October to May. Immunity is formed within a few weeks: a pre-vaccination with the Sovigripp vaccine will allow for timely and effective prevention of the disease.
The virus is mutating. Where are the guarantees that the vaccine will protect against it?
The composition of the Sovigripp vaccine is updated annually in accordance with WHO recommendations and the EU decision on the composition of influenza vaccines for the season. The vaccine protects against the three most current (dangerous) varieties (strains) of the influenza virus this season, which are determined thanks to the work of WHO specialists, as well as research institutes that transmit data on current strains on each continent in order to formulate a forecast and epidemic. It’s time to release effective and relevant vaccines, which include Sovigripp.
My grandmother just retired after reaching the age of 55, can she be vaccinated against the flu with the Sovigripp vaccine?
Yes, clinical studies have proven the effectiveness, weak reactivity and good tolerability of the Sovigripp vaccine by people under and after 60 years of age. Your grandmother can get vaccinated: Vaccination is the most effective and cost-effective measure to fight infections, including the flu.
Do I need to get a flu shot every year?
It is recommended to be vaccinated annually - different vaccines are created every year based on the viruses expected in the coming season. They do not protect against diseases caused by other viruses not contained in this vaccine. In addition, according to statistics, vaccinated people, even if they get sick, tolerate the flu much easier, and complications occur less frequently.
Why get vaccinated?
The vaccine does not protect 100% from the flu, but it minimizes both the likelihood of illness and the possibility of complications. In addition, vaccination alleviates the course of the disease.
The effectiveness of immunization against influenza depends on several factors - the quality of the vaccine, the characteristics of each person’s body and the epidemiological situation in the place of residence.
The fact that even a vaccinated person can get the flu is explained by the fact that vaccination is carried out against one influenza virus, and the person gets sick with another type of virus.
Possible complications in children include pneumonia, otitis media and croup. Pneumonia is also the most common complication of the disease in older people. Children may also develop Reye's syndrome (severe vomiting and confusion that can lead to coma).
In an adult, influenza can lead to complications such as bacterial pneumonia, viral pneumonia, febrile seizures, myocarditis, exacerbation of pulmonary diseases (bronchitis), myositis, meningitis or encephalitis.
Who shouldn't get a flu shot?
Before getting a flu vaccination, all allergy sufferers should consult a doctor, especially if you have an allergic reaction to eggs, as well as if there is a possibility of a reaction to any component of the vaccine.
At the same time, residual effects after an acute respiratory infection (cough, runny nose) are not a reason not to get vaccinated at all or to postpone the date of vaccination.
Source: https://www.sovigripp.ru
FLU. Read more…
Drug interactions
Grippol® plus can be used simultaneously with inactivated and live vaccines of the National Preventive Vaccination Calendar (except for BCG and BCG-M) and inactivated vaccines of the preventive vaccination calendar for epidemic indications (except for rabies). The vaccine can be administered against the background of basic therapy for the underlying disease.
Come get vaccinated at MAMARADA. A full range of vaccines for children and adults, family vaccinations - at a special price!