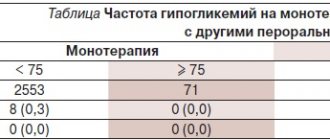
Analgin
When treating patients receiving cytostatic drugs, as well as children under 5 years of age, treatment with metamizole sodium should be carried out only under the supervision of a physician.
Anaphylactic/anaphylactoid reactions
When choosing a method of drug administration, it should be taken into account that parenteral administration is associated with a higher risk of anaphylactic/anaphylactoid reactions.
An increased risk of developing hypersensitivity reactions to metamizole sodium may be due to the following conditions:
- analgesic bronchial asthma, especially with concomitant polypous rhinosinusitis;
- chronic urticaria;
- alcohol intolerance (increased sensitivity to alcohol), against the background of which, even when taking a small amount of certain alcoholic beverages, patients experience sneezing, lacrimation and severe redness of the face. Alcohol intolerance may indicate previously unidentified aspirin asthma syndrome;
- intolerance or hypersensitivity to dyes (for example, tartrazine) or preservatives (for example, benzoate).
Before using metamizole sodium, it is necessary to conduct a thorough survey of the patient in order to determine medical history.
If a risk of developing anaphylactic reactions is identified, administration is possible only after a thorough assessment of the ratio of the expected benefit to the possible risk of using the drug. In the case of using metamizole sodium in such patients, strict medical supervision of their condition is necessary and it is necessary to have means to provide them with emergency assistance in the event of the development of anaphylactic/anaphylactoid reactions.
Anaphylactic shock may occur in predisposed patients, so metamizole sodium should be administered with caution to patients with asthma or atopy. Patients who experience anaphylactoid reactions in response to the use of metamizole sodium are also at risk of developing them in response to the use of other non-narcotic analgesics/non-steroidal anti-inflammatory drugs (NSAIDs).
Patients who experience anaphylactic or other immune-mediated reactions (eg, agranulocytosis) in response to the use of metamizole sodium are also at risk of developing them with the use of other pyrazolones and pyrazolidines.
Severe skin reactions
Life-threatening skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been described with the use of metamizole sodium. If symptoms of SJS and TEN appear, including progressive skin rash, often with blisters or mucosal lesions, treatment with metamizole sodium should be stopped immediately and should not be continued in this category of patients. Patients should be aware of the symptoms of these diseases. They should be carefully monitored for skin reactions, especially during the first days of treatment.
Agranulocytosis
With long-term use (more than 7 days), it is necessary to monitor the peripheral blood picture. While taking metamizole sodium, agranulocytosis may develop. It occurs very rarely, lasts at least a week, is not dose-dependent, can be severe, life-threatening and, in some cases, fatal. Therefore, if you identify symptoms such as an unmotivated rise in temperature, chills, sore throat, difficulty swallowing, stomatitis, erosive and ulcerative lesions of the oral cavity, vaginitis or proctitis, a decrease in the number of neutrophils in the peripheral blood less than 1500/mm3, you must immediately contact doctor and stop the drug.
Pancytopenia
If pancytopenia develops, treatment should be stopped immediately; complete blood count parameters should be monitored until they return to normal. All patients should be aware that pathological changes in the blood may be accompanied by the appearance of symptoms such as general malaise, infections, persistent fever, hematoma formation, bleeding, pallor, while taking metamizole sodium, which requires immediate consultation with a doctor.
Isolated hypotensive reactions
Metamizole sodium may cause isolated hypotensive reactions. These reactions may be dose-dependent. The risk of such reactions is also increased in: pre-existing hypotension, decreased circulating blood volume or dehydration, unstable hemodynamics or acute circulatory disorders (for example, in patients with myocardial infarction or trauma), in patients with fever. In this regard, such patients should undergo detailed diagnostics and be closely monitored. In order to reduce the risk of hypotensive reactions, preventive measures (hemodynamic stabilization) may be required.
In patients in whom lowering blood pressure should be avoided at all costs (for example, with severe ischemic heart disease or significant cerebral artery stenosis), metamizole sodium should only be used with careful monitoring of hemodynamic parameters.
Abdominal pain
It is unacceptable to use the drug to relieve acute abdominal pain (until the cause is determined).
Liver and kidney dysfunction
In patients with impaired liver or kidney function, it is recommended to avoid taking metamizole sodium in high doses due to a decrease in the rate of elimination of the drug. The drug contains sodium, which should be taken into account by people on a low sodium diet.
Rules for administering the drug
Intravenous administration should be carried out very slowly (no more than 1 ml per minute) so that at the first signs of anaphylactic/anaphylactoid reactions the administration can be stopped, as well as to minimize the occurrence of individual hypotensive reactions. For intramuscular administration, it is necessary to use a long intramuscular needle.
Buy Analgin tablets 500 mg No. 20 in pharmacies
Analgin Buy Analgin in pharmacies
DOSAGE FORMS tablets 500 mg
MANUFACTURERS
PFC update (Russia)
GROUP Analgesics-antipyretics - pyrazolone derivatives
COMPOSITION Active ingredient: Metamizole sodium.
INTERNATIONAL NON-PROPENTED NAME Metamizole sodium
SYNONYMS Analgin-Akos, Analgin-Rusfar, Analgin-UBF, Analgin-Ultra, Baralgin M, Metamizole sodium, Nebagin, Nobol, Optalgin-Teva, Spazdolzin
PHARMACOLOGICAL ACTION Anti-inflammatory, analgesic, antipyretic. Inhibits the activity of cyclooxygenase, reduces the formation of endoperoxides, bradykinins, some prostaglandins, free radicals, and inhibits lipid peroxidation. It prevents the conduction of painful extra- and proprioceptive impulses along the Gaulle and Burdach bundles, increases the threshold of excitability of the thalamic centers of pain sensitivity, and increases heat transfer. When taken orally, it is quickly and completely absorbed. Destroyed in the liver. Excretion passes through the kidneys. The action develops after 20-40 minutes and reaches a maximum after 2 hours.
INDICATIONS FOR USE Arthralgia, rheumatism, chorea, pain: headache, dental, menstrual, neuralgia, sciatica, myalgia, colic (renal, hepatic, intestinal), pulmonary infarction, myocardial infarction, dissecting aortic aneurysm, thrombosis of the great vessels, inflammatory processes ( pleurisy, pneumonia, lumbago, myocarditis), trauma, burns, decompression sickness, herpes zoster, tumors, orchitis, pancreatitis, peritonitis, esophageal perforation, pneumothorax, post-transfusion complications, priapism; febrile syndrome in acute infectious, purulent and urological diseases (prostatitis), insect bites (mosquitoes, bees, gadflies, etc.).
CONTRAINDICATIONS Hypersensitivity, inhibition of hematopoiesis (agranulocytosis, cytostatic or infectious neutropenia), severe dysfunction of the liver or kidneys, prostaglandin bronchial asthma, hereditary hemolytic anemia associated with deficiency of glucose-6-phosphate dehydrogenase, pregnancy, breastfeeding (stop during treatment).
SIDE EFFECTS Granulocytopenia, agranulocytosis, thrombocytopenia, hemorrhages, hypotension, interstitial nephritis, allergic reactions (including Stevens-Johnson, Lyell syndromes, bronchospasm, anaphylactic shock).
INTERACTION The effect is enhanced by barbiturates, codeine, histamine H2 blockers, anaprilin (slows down inactivation). Sarcolysin and Mercazolil increase the likelihood of developing leukopenia. Increases the hypoglycemic activity of oral antidiabetic drugs (released from binding to blood proteins), the sedative activity of alcohol, and reduces the concentration of cyclosporine in plasma.
METHOD OF APPLICATION AND DOSAGE Orally after meals, 250-500 mg 2-3 times a day, maximum single dose - 1 g, daily dose - 3 g. Usual dose for children 2-3 years old - 50-100 mg, 4-5 years old - 100-200 mg, 6-7 years - 200 mg, 8-14 years - 250-300 mg 2-3 times a day.
OVERDOSE Symptoms: hypothermia, severe hypotension, palpitations, shortness of breath, tinnitus, nausea, vomiting, weakness, drowsiness, delirium, impaired consciousness, convulsions; the development of acute agranulocytosis, hemorrhagic syndrome, acute renal and liver failure is possible. Treatment: induction of vomiting, transtube gastric lavage, administration of saline laxatives, activated charcoal and forced diuresis, alkalization of the blood, symptomatic therapy aimed at maintaining vital functions.
SPECIAL INSTRUCTIONS Medical supervision is required. Regular long-term use is not recommended due to myelotoxicity. Do not use it to relieve acute abdominal pain (until the cause is determined). When prescribed to patients with acute cardiovascular pathology, careful monitoring of hemodynamics is necessary. Use with caution in patients with systolic blood pressure below 100 mmHg, with anamnestic indications of kidney disease (pyelonephritis, glomerulonephritis) and with a long history of alcoholism. When using metamizole, urine may turn red due to the release of a metabolite.
STORAGE CONDITIONS List B. In a dry place protected from light.
Analgin
Analgin
Registration number: LS – 002585
Trade name of the drug: Analgin
International nonproprietary name: Metamizole sodium
Chemical rational name:
Sodium 1-Phenyl-2,3-dimethyl-4-methylaminopyrazolone-5-N-methanesulfonate
Dosage form: tablets
Compound
active substance: metamizole sodium - 500 mg
excipients: refined sugar (sucrose), potato starch, calcium stearate 1-water, talc.
Description
Tablets are white or white with a slightly yellowish tint, flat-cylindrical in shape, scored and chamfered.
Pharmacotherapeutic group
Analgesic non-narcotic drug.
ATX code: [ N 02 BB 02 ]
Pharmacological properties
It has analgesic, antipyretic and weak anti-inflammatory effects, and is a derivative of pyrazolone.
Pharmacodynamics
Analgin non-selectively blocks cyclooxygenase and reduces the formation of prostaglandins from arachidonic acid, and prevents the conduction of painful extra- and proprioceptive impulses. Analgin has a weak anti-inflammatory effect, which causes little effect on water-salt metabolism (sodium and water retention) and the gastrointestinal mucosa. It has an antispasmodic effect on the smooth muscles of the urinary and biliary tract. The action develops 20-40 minutes after ingestion.
Pharmacokinetics
It is well and quickly absorbed from the gastrointestinal tract, which ensures rapid development of the clinical effect. When taken in therapeutic doses, it penetrates into mother's milk.
Maximum plasma concentration is achieved 1-1.5 hours after oral administration. In the intestinal wall it is hydrolyzed to form the active metabolite, 4-methyl-amino-antipyrine, which in turn is metabolized to 4-formyl-amino-antipyrine and other metabolites. The level of binding of the active metabolite to proteins is 50-60%. Excretion of metabolites occurs through the kidneys. In addition, metabolites are excreted in breast milk.
Indications for use
Feverish syndrome against the background of infectious and inflammatory diseases, headaches of various origins, pain syndrome of mild and moderate severity: neuralgia, myalgia, arthralgia, biliary colic, intestinal colic, renal colic, trauma, radiculitis, myositis, postoperative pain syndrome, algodismenorrhea.
Contraindications
Hypersensitivity, inhibition of hematopoiesis (agranulocytosis, neutropenia), liver and/or renal failure, hereditary hemolytic anemia associated with deficiency of glucose-6-phosphate dehydrogenase and other types of anemia, “aspirin” asthma, leukopenia, pregnancy (especially in the first trimester and in the last 6 weeks), lactation period, children up to 8 years of age.
Carefully
The drug should be taken with caution in case of kidney diseases (pyelonephritis, glomerulonephritis - including a history), with moderately severe liver and kidney disorders, bronchial asthma, a predisposition to the development of arterial hypotension, and long-term alcohol abuse.
Directions for use and doses
Orally, after meals, adults are prescribed 250-500 mg 2-3 times a day, the maximum single dose is 1000 mg, the daily dose is 3000 mg. Single doses for children 8-14 years old - 250-300 mg, frequency of administration 2-3 times a day. The duration of use without consulting a doctor is no more than 5 days.
Side effect
From the urinary system: impaired renal function, oliguria, anuria, proteinuria, interstitial nephritis, red staining of urine.
Allergic reactions: urticaria (including on the conjunctiva and mucous membranes of the nasopharynx), angioedema, in rare cases - malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), bronchospastic syndrome, anaphylactic shock.
From the hematopoietic organs: agranulocytosis, leukopenia, thrombocytopenia.
Other: lowering blood pressure.
Overdose
Symptoms: nausea, vomiting, gastralgia, oliguria, hypothermia, decreased blood pressure, tachycardia, shortness of breath, tinnitus, drowsiness, delirium, impaired consciousness, acute agranulocytosis, hemorrhagic syndrome, acute renal and/or liver failure, convulsions, respiratory muscle paralysis .
Treatment: gastric lavage, saline laxatives, activated charcoal, forced diuresis, hemodialysis, with the development of convulsive syndrome - intravenous administration of diazepam and fast-acting barbiturates.
Interaction with other drugs: Concomitant administration with other non-narcotic analgesics, tricyclic antidepressants, contraceptive hormonal drugs and allopurinol may lead to increased toxicity; sedative and anxiolytic drugs enhance the analgesic effect of metamizole sodium. Metamizole sodium enhances the effects of alcohol; simultaneous use with chlorpromazine or phenothiazine can lead to the development of severe hyperthermia. Radiocontrast drugs, colloidal blood substitutes and penicillin should not be used during treatment with metamizole sodium.
With simultaneous administration of cyclosporine, the concentration of the latter in the blood decreases. Metamizole sodium, displacing oral hypoglycemic drugs, indirect anticoagulants, corticosteroids and indomethacin from protein binding, increases their activity. Phenylbutazone, barbiturates and other inducers of microsomal liver oxidation, when administered simultaneously, reduce the effectiveness of metamizole sodium.
special instructions
When treating children under 8 years of age and patients receiving cytotoxic drugs, Analgin should only be taken under medical supervision.
Patients with atopic bronchial asthma and hay fever have an increased risk of developing allergic reactions. While taking Analgin, agranulocytosis may develop, and therefore, if an unmotivated rise in temperature, chills, sore throat, difficulty swallowing, stomatitis is detected, as well as with the development of vaginitis or proctitis, immediate discontinuation of the drug is necessary. With long-term use, it is necessary to monitor the peripheral blood picture.
It is not permissible to use it to relieve acute abdominal pain (until the cause is determined).
Release form
Tablets 500 mg. 10 tablets in blister or cell-free packaging. 2 or 3 blister packs along with instructions for use are placed in a cardboard pack. It is allowed to place contour cell or cell-free packages with an equal number of instructions for use in a box made of cardboard.
Conditions for dispensing from pharmacies
Without a doctor's prescription.
Storage conditions:
List B. In a dry place, protected from light, at a temperature not exceeding 25 ° C.
Best before date:
5 years. Do not use after the date indicated on the package.
Units:
pack