Metabolic syndrome (MS) is a complex metabolic disorder based on insulin resistance and compensatory hyperinsulinemia.
This symptom complex is a risk factor for the development of a large number of different diseases.
In the absence of timely detection, lifestyle correction and adequate treatment, metabolic syndrome can lead to the development of such serious ailments in the patient as type 2 diabetes mellitus, atherosclerosis, arterial hypertension and coronary heart disease.
Most often, metabolic syndrome is diagnosed in men aged 30+, but in recent decades the number of recorded cases of pubertal insulin resistance has been growing - that is, similar health problems arise in very young patients.
Most likely, the occurrence of metabolic syndrome in children and adolescents is explained by poor nutrition. This is a truly global problem - according to WHO, today more than 41 million preschool children in the world are overweight.
Symptoms of metabolic syndrome in women often appear after 50 years of age (postmenopausal period); with age, the number of patients only grows.
Early diagnosis of metabolic disorders in combination with the recommendations of an endocrinologist makes it possible to eliminate or reduce the severity of the main manifestations of this syndrome.
Metabolic syndrome: causes
Normally, nutrients entering the body during the process of aerobic (with the participation of oxygen) and anaerobic (without the participation of oxygen) glycolysis should be oxidized to carbon dioxide, water, ammonia, then the body can easily remove these products and begin a new cycle of processing nutrients without harm to organs and systems.
However, an unfavorable environmental situation, unhealthy food, overwork, stress, and hereditary factors lead to incomplete, intermediate oxidation and the formation of small and medium-sized hydrocarbon molecules that accumulate in cells and intercellular substance, deposited on cell membranes and vessel walls.
All this entails disruption of fluid circulation in the body, disruption of the supply of nutrients and oxygen to cells, which ultimately further disrupts metabolism. Also, histamine, serotonin, catecholamines and many other biologically active substances formed in various diseases and injuries that occurred in life accumulate in the body.
It is quite difficult, and in most cases impossible, for the body to cope with so many toxins, which over time leads to the development of various diseases.
Metabolic syndrome (MS) is a symptom complex that combines disorders of lipid and carbohydrate metabolism and their pathological manifestations. The latter are the cause of atherosclerosis, cardiovascular diseases and type 2 diabetes. Data from epidemiological studies have shown that the frequency of MS increases with age, increasing sharply in people over 50 years of age. The leading role of visceral adipose tissue in the development of insulin resistance has been determined. However, at present, the phenomenon of insulin resistance has not been sufficiently studied, and the etiological factors for the development of the pathological process have not been identified. It becomes obvious that the metabolic disorders underlying atherosclerosis and type 2 diabetes mellitus must be considered as a subject of gerontology.
Metabolic syndrome: pathogenesis and geriatric aspects of the problem
Metabolic syndrome is a number of certain criteria reflecting abnormalities in lipid and glucose metabolism and its pathological manifestations. These abnormalities are considered to be a reason for atherosclerosis, cardiovascular diseases and diabetes mellitus type 2. Data from epidemiological studies have shown that the incidence of metabolic syndrome increases with age, increasing sharply in patients older than 50 years. It was defined the leading role of visceral adipose tissue in the development of insulin resistance. The phenomenon of insulin resistance is not studied enough, not identified etiological factors in the development of pathological process. Metabolic abnormalities that underlie atherosclerosis and diabetes type 2 should be regarded as a subject of gerontology.
The study of metabolic syndrome (MS) has gone through a complex historical path. Since ancient times, it has been known that overeating and wine abuse were often combined with obesity and gout, which led to the development of stroke. This phenomenon was most common among representatives of high society. In 1923, the Swedish physician Kylin described a syndrome called “hypertension-hyperglycemia-hyperuricemia.” At the same time, Soviet scientist G.F. Lang pointed out the close connection between arterial hypertension (AH) and obesity, carbohydrate metabolism disorders and gout. Engelhardt and Wagner (1950) called obesity an integral part of a triad, the other components of which are gout and diabetes. In 1981, based on epidemiological and pathophysiological studies, East German scientists M. Hanefeld and W. Leonardt put forward the classical theory of MS (Das metabolische Syndrom), which included obesity, hypertension, hyperlipidemia, gout, and type 2 diabetes mellitus. Hyperinsulinemia (HI) has been identified as an important link in the development of atherosclerosis. However, the proposals of scientists from Eastern European countries have not found application in the leading medical circles of the world. In 1988, the American scientist G. Reaven, in the famous Banting lecture, stated that insulin resistance is the main pathogenetic link of MS, which he called syndrome X. In 1989, N. Kaplan introduced the term “deadly quartet,” emphasizing the increased risk of mortality from coronary heart disease (CHD). ) in this category of patients [1].
According to the literature, MS has 7 synonyms and more than 20 scientists claim primacy in the development of this theory. An analysis of fundamental works on the problem of MS showed that the contribution of these authors to the formation of modern ideas about this pathology is undeniably important. However, in almost all publications devoted to MS, G. Reaven is recognized as the founder of the theory of MS, and his article “The Role of Insulin Resistance in Human Diseases” is the most cited work [1].
The current demographic situation is characterized by a rapid increase in the population of older people in the world, including in Russia. Currently, approximately 12-15% of the planet's population are people over 65 years of age, and by 2021 their number will at least double. About 30% (1.7 billion people) of the world's inhabitants are overweight. It is known that the presence of central obesity, one of the main components of MS, significantly increases the risk of developing the following cardiovascular complications: coronary heart disease - 2-4 times, myocardial infarction - 6-10 times, cerebral stroke - 4-7 times . In this case, mortality increases by 2.3 times [2]. In this regard, MS is an extremely pressing problem of modern medicine. Metabolic syndrome is understood as a complex of pathologically interrelated metabolic disorders of carbohydrate and lipid metabolism, insulin resistance, visceral obesity and arterial hypertension. WHO experts described MS as a “pandemic of the 21st century” [3]. Data from epidemiological studies have shown that the frequency of MS increases gradually as the body ages, increasing sharply in people over 50 years of age. The frequency of MS is 20-40%, increases with age in both men and women, reaching its maximum value in the age group of 60-69 years. The trend towards a continuous increase in the number of people with MS, observed in old and senile age, seems to “freeze” at o [4]. Currently, MS is included in the list of diseases associated with age.
Obesity is an independent risk factor for cardiovascular complications, as well as a possible trigger for the development of other cardiovascular diseases, such as hypertension. Long-term arterial hypertension leads to the development of insulin resistance (IR) and diabetes mellitus (DM), and the addition of DM leads to the progression of hypertension and further significantly increases the risk of developing cardiovascular complications. Obesity is also a risk factor for the development of type 2 diabetes. This nosological form, as an independent metabolic disease, is characterized by a chronic course and requires lifelong treatment, without which it steadily progresses. The combination of the central type of obesity, essential hypertension, IR, disorders of carbohydrate and lipid metabolism and the pathogenetic connection between them served as the basis for identifying them as an independent syndrome.
To date, there is no consensus on the root cause of metabolic disorders in the pathogenesis of MS. Abdominal obesity may be the primary factor causing the appearance of components of the metabolic syndrome and combining them into a single complex [5, 6, 7]. It has been proven that abdominal fat easily undergoes lipolysis, for example, under stress. At the same time, a significant amount of non-esterified (free) fatty acids (NEFA) is released into the bloodstream. Through the portal vein, excess NEFAs enter the liver, where they are utilized.
One way to utilize NEFAs, massively entering the liver during lipolysis of abdominal fat, is their conversion into glucose through the processes of gluconeogenesis. As a result, the liver secretes excess glucose into the bloodstream. This causes hyperglycemia, in response to which the liver's removal of insulin from the bloodstream is reduced, contributing to hyperinsulinemia. In parallel, excess glucose in the blood contributes to the development of tissue insulin resistance.
The second way to utilize NEFAs entering the liver is the synthesis of triglycerides (TG). There is an increase in the synthesis of the main protein of low-density lipoproteins - apolipoprotein (apo) B. At the same time, the assembly and secretion of very low-density lipoproteins (VLDL) into the blood increases [8]. As a result, hyperlipidemia develops with an increased concentration of TG in the blood plasma due to VLDL, which is often accompanied by a moderately increased level of cholesterol (C), mainly due to an increase in the concentration of low-density lipoproteins (LDL). This is due to the fact that secreted VLDL in the bloodstream is exposed to the action of two key lipolytic enzymes: lipoprotein lipase and hepatic lipase. After exposure to the first, large VLDL particles become smaller and turn into intermediate-density lipoprotein (IDL) particles, which, upon contact with hepatic lipase, turn into LDL particles, represented predominantly by a subfraction of small dense particles. They are a characteristic feature of the lipid spectrum in metabolic syndrome and have a highly atherogenic potential. Due to low affinity for LDL receptors and prolonged circulation in the systemic circulation, small and dense LDL are oxidized, forming chemically modified LDL, which are easily and uncontrollably taken up by macrophages. In the fate of an atherosclerotic plaque, the latter play an important role, contributing to the formation of a malignant plaque with an extensive soft lipid core and a thin connective tissue cap, which easily ruptures and entails thrombosis. It is atherothrombosis that causes the development of acute clinical episodes of coronary heart disease (CHD) and diseases associated with cerebral or peripheral localization of the atherosclerotic process.
The development of atherosclerotic vascular lesions is promoted by endothelial dysfunction. One of the factors damaging the endothelium is dyslipoproteinemia (DLP), with an increase in the content of low-density lipoproteins in the blood plasma, transporting cholesterol into cells, and a decrease in the content of high-density lipoproteins (HDL), which transport cholesterol to the liver, where it is catabolized to bile acids. Such DLP is characteristic of metabolic syndrome. The activity of lipoprotein lipolysis and LDL uptake receptors determine the type of hyperlipidemia. As a rule, this is combined hyperlipidemia, i.e. with an increase in the level of TG and cholesterol, which is accompanied by a reduced level of HDL cholesterol. There may be several reasons for low levels of HDL cholesterol, including: a) reduced synthesis of their main proteins apo AΙ and AΙΙ; b) insufficient transfer of VLDL surface fragments containing non-esterified cholesterol and phospholipids to HDL, with a deficiency of VLDL lipoprotein lipolysis and hypertriglyceridemia; c) active transfer of cholesterol esters from HDL to VLDL in exchange for their TG; d) the active effect of hepatic lipase on HDL particles enriched with triglycerides, as a result of which TG and phospholipids are broken down in them, and small remnants of HDL particles (HDL-3 subfraction) return to the bloodstream without effective removal of cholesterol to the liver. Regardless of the cause, dysfunction of HDL to ensure the outflow of cholesterol into the liver contributes to the development of the atherosclerotic process [9].
Currently, insulin resistance (IR) plays an increasingly significant role in the development of MS. In the development of IR, both the factor of genetic predisposition and certain lifestyle features are important: excess fat consumption and decreased physical activity, the use of various medications that can cause disturbances in carbohydrate metabolism, and an increase in the number of stressful situations in the lives of patients. The basis of the genetic factors for the development of IR is a violation of the receptor and post-receptor mechanisms of insulin signal transmission. The cellular mechanisms of insulin resistance may vary between tissues. For example, a decrease in the number of insulin receptors is found on adipocytes and, to a lesser extent, in muscle cells. Impaired translocation of intracellular glucose transporters, GLUT-4, to the plasma membrane is most pronounced in adipocytes. In the development of insulin sensitivity disorders, mutations in the insulin receptor substrate (ISR-1) genes, b3-adrenergic receptors, tumor necrosis factor-a, and a decrease in the membrane concentration and activity of intracellular glucose transporters, GLUT-4, in muscle tissue may be important [10]. Despite significant advances in uncovering the mechanisms of action of insulin, there is still no unambiguous understanding of the causes of IR development at the molecular level.
It has been established that IR and compensatory HI affect a number of mechanisms regulating blood pressure (BP). Hyperinsulinemia has a prohypertensive effect through an increase in the reabsorption of sodium and water by the kidneys, stimulation of the centers of the sympathetic nervous system and activation of Na+/H+ exchange in vascular smooth muscle cells, which promotes the accumulation of Na+ and Ca+ ions in them and increases sensitivity to the pressor effects of catecholamines and angiotensin II . Stimulation of the local renin-angiotensin vascular system by insulin causes the growth and proliferation of smooth muscle cells and promotes the development of remodeling processes (hypertrophy of the muscular lining of blood vessels, reduction of the internal diameter), which is a factor in stabilizing elevated blood pressure levels [11].
Activation of the renin-angiotensin system, development of vascular remodeling processes, restructuring of kidney function and baroreceptor apparatus contribute to the “fixation” of elevated blood pressure levels. However, IR may contribute during hypertension and at later stages of its development. Activation of the sympatho-adrenal system under the influence of GI and increased levels of FFA leads to a disruption of the circadian rhythm of blood pressure with its insufficient decrease at night, i.e. to the development of nocturnal hypertension. But there is also an inverse relationship: the development of MS due to a long course of essential hypertension, which leads to a decrease in peripheral blood flow and the development of IR.
The endocrine role of the vascular endothelium in the genesis and development of hypertension is no less important, since the endothelium is the main target organ in IR conditions. Animal experiments have shown that the administration of insulin leads to increased production of vasodilators and a decrease in blood pressure, but under IR conditions the opposite occurs: the endothelium increases production of vasoconstrictors - endothelin, thromboxane AII, prostaglandin FII, and decreases the secretion of vasodilators - prostacyclin and the most powerful of them - nitric oxide .
In addition, a new theory of the pathogenesis of hypertension in obesity has recently been discussed, associated with hyperleptinemia in these patients. Leptin is a hormone synthesized by adipocytes of visceral adipose tissue. Leptin concentrations depend on total body weight and body fat percentage. It has been proven that leptin resistance in obese patients is in some cases associated with a genetic defect in its receptors [12]. Leptinemia correlates with most anthropometric parameters. Thus, in women there is a closer relationship between leptin levels and body mass index (BMI), including postmenopause [13]. In men, leptinemia correlates with waist circumference, hip circumference, and waist/hip index (W/H), but for women this issue remains controversial.
Many studies have shown that in the liver, leptin can inhibit the effect of insulin on gluconeogenesis by influencing the activity of the rate-limiting enzyme of gluconeogenesis. Some studies have found that leptin may have an inhibitory effect on insulin receptor substrate tyrosine phosphorylation (IRS-1) in muscle tissue [14].
Insulin is an important regulator of leptin secretion. Adipocytes produce leptin in response to increased insulin levels after a meal. Leptin concentration is determined by insulinemia not only after meals, but also on an empty stomach [15].
Both insulin and leptin regulate satiety. Leptin regulates the feeling of satiety at the level of the arcuate nucleus of the hypothalamus, from which a rich network of axons extends to the paraventricular nucleus. Stimulation of the paraventricular nucleus leads to activation of a number of sympathetic nerves (renal, adrenal and visceral) and an increase in plasma catecholamine concentrations. Leptin levels are closely related to blood pressure, angiotensin and norepinephrine levels. A study of the causal relationship between hyperleptinemia and hypertension conducted in Japan (data analysis of almost 2000 men) showed that in obese individuals, blood pressure levels, leptin, insulin and norepinephrine concentrations were higher than in hypertensive patients with normal body weight. At the same time, an increase in blood pressure against the background of an increase in body weight correlated more closely with an increase in the concentration of norepinephrine than insulin, i.e. increased activity of the sympathetic nervous system (SNS) preceded the development of HI. In obese individuals, a dependence of blood pressure level on leptin concentration was also revealed, which was absent in the group of hypertensive patients with normal body weight. Thus, the data obtained indicate the existence of a causal relationship between hyperleptinemia, increased activity of the SNS and hypertension in obese patients [16]. The relationship between blood leptin levels and insulin sensitivity (the higher the leptinemia, the lower the insulin sensitivity) indicates that hyperleptinemia is an integral part of the metabolic syndrome.
If the pathogenesis, clinical manifestations and, accordingly, treatment tactics in relation to the glycemic profile in people with MS in young and middle age are relatively predictable, then the gerontological aspects of this problem have their own characteristics.
After 50 years, for every subsequent 10 years, fasting blood glucose increases by 0.055 mmol/l, and postprandial blood glucose increases by 0.5 mmol/l. A decrease in tissue sensitivity to insulin (insulin resistance) is the main mechanism leading to disturbances in carbohydrate metabolism in overweight people. In elderly and senile people, using a hyperglycemic clamp, a decrease in the sensitivity of peripheral tissues to insulin and, accordingly, a decrease in glucose uptake by peripheral tissues was revealed. This defect is mainly detected in people over 60 years of age with excess body weight. Old age brings with it many additional factors that aggravate existing insulin resistance. This includes low physical activity as a result of the presence of a number of chronic somatic conditions, and a decrease in muscle mass (the main peripheral tissue that utilizes glucose), and abdominal obesity (increases by the age of 70 years, then, as a rule, decreases). Decreased insulin secretion is the main defect underlying the development of type 2 diabetes in non-obese individuals. As is known, insulin secretion in response to intravenous glucose occurs in two stages (two phases): the first phase is rapid intense insulin secretion, lasting the first 10 minutes; the second phase is longer (up to 60-120 minutes) and less pronounced. The first phase of insulin secretion is necessary for effective control of postprandial glycemia. The vast majority of researchers have identified a significant decrease in the first phase of insulin secretion in elderly people without excess body weight. This is probably what is responsible for such a pronounced increase in postprandial glycemia (by 0.5 mmol/l) every decade after 50 years of age. Numerous studies conducted in the 1980s and 1990s showed that hepatic glucose production does not change significantly with age. The blocking effect of insulin on glucose production by the liver also does not decrease. Therefore, changes in hepatic glucose metabolism may not underlie the pronounced age-related changes in glucose tolerance. Indirect evidence indicating normal glucose production by the liver in elderly and senile people is the fact that fasting glycemia (largely dependent on the release of glucose by the liver at night) changes very little with age [17].
Thus, in old age, glucose metabolism is determined by two main factors: tissue sensitivity to insulin and insulin secretion. The first factor, insulin resistance, predominates in obese individuals over 60 years of age. The second factor—reduced insulin secretion—dominates in elderly and senile people without excess body weight. Knowledge of the basic mechanisms of development of type 2 diabetes allows for a differentiated approach to prescribing therapy in geriatric practice.
In the last decade, a “new” pathophysiological component of MS—urate metabolism—has attracted increased interest. Serum uric acid (UA) levels increase along with increases in uric acid components, even when adjusted for several confounding factors such as age, sex, changes in creatinine clearance, alcohol, and diuretic use. In a population-based study, the mean serum UA level increased from 4.6 mg/dL among patients without MS components to 5.9 mg/dL among patients with three components. The strongest correlation of uricemia was determined with waist circumference [18, 19, 20].
The increase in sUA levels in patients with IR and hyperinsulinemia is due to the ability of insulin to slow down the clearance of sUA in the proximal tubules of the kidneys. This mechanism is considered as one of the possible explanations for the development of hyperuricemia (HU) and gout in the presence of MS components [21, 22, 23]. On the other hand, the primary damaging effect of HU on the kidneys leads to the development of urate nephropathy and, as a consequence, impaired excretion of sUA, the development of gouty arthritis and the aggravation of visceral lesions. As an option to substantiate the increased synthesis of UA in MS, it is assumed that there is an increase in fructose consumption. The process of phosphorylation of fructose in the liver leads to a decrease in ATP production, which is ultimately reflected in the form of an increase in the synthesis of UA. It has been suggested that the widespread sale of high-fructose corn syrup (which is more stable and cheaper than sugar) has contributed significantly to the incidence of metabolic syndrome seen in the US population in recent years [19, 20, 22].
Back in 1967, Myers A., using a population of 6,000 people, showed that there is a direct correlation of excess weight with the incidence of hyperuricemia and the development of coronary diseases. The study, based on data from the Multivariable Risk Factors Study (MRFIT), reported that gout was associated with a 26% increased risk of acute myocardial infarction. Thus, an increase in serum UA levels among patients with obesity, hypertension or impaired glucose tolerance (IGT) with high cardiovascular risk (especially in those with coronary artery disease) can be considered as a marker of exacerbation of cardiovascular diseases (CVD), coronary artery disease failure and oxidative stress in the cardiovascular system.
Currently, studies have been published confirming that MS is a factor predisposing to the development of cardiac arrhythmias. In persons with MS, compared with the control group, there is a decrease in heart rate variability (HRV), the rhythmogram is characterized by a decrease in RR intervals and their monotony, an increase in the number of areas with low variability and a decrease in the integral assessment of normal variability are detected. Such dynamics of indicators characterize the development of autonomic dysfunction (hypersympathicotonia) and cardiac autonomic neuropathy in MS, which leads to electrical instability of the myocardium, silent ischemia and arrhythmia. As is known, it is the autonomic nervous system that plays an important role in the initiation of malignant ventricular arrhythmias. The likelihood of their occurrence is usually associated with an increase in the tone of the sympathetic and a decrease in the tone of the parasympathetic nervous system. In the work of Park SK et al. (2006) examined 423 elderly men (Normative Aging Study), of whom 32% were diagnosed with MS. Among other research methods, such spectral components of HRV as HF, LF and the LF/HF ratio were assessed. It turned out that in patients with MS, the high-frequency HF component, which characterizes the tone of the parasympathetic nervous system, is reduced, the low-frequency LF component, which characterizes the tone of the sympathetic nervous system, is increased, and the LF/HF ratio, which characterizes the balance of the sympathetic and parasympathetic nervous system, is correspondingly changed [24].
With aging, reflex effects on the cardiovascular system are weakened, and disintegration of various levels of autonomic regulation of cardiac activity is observed. At the same time, parasympathetic influences on the heart weaken at a faster pace. As a result, in older people, against the background of a general decrease in autonomic tone, a relative predominance of sympathetic regulation is formed [25]. Maximum stimulation of the sympathetic nervous system is observed in individuals with IR, hyperinsulinemia and obesity.
Thus, the combination of MS components is due to metabolic and physiological relationships between them, which potentiates their pathogenicity not only in relation to non-insulin-dependent diabetes mellitus, but also in relation to coronary heart disease and other diseases caused by atherosclerosis.
Isolation of MS is of great clinical importance: since, on the one hand, this condition is reversible, i.e. with appropriate treatment, it is possible to achieve disappearance or at least a decrease in the severity of its main symptoms; on the other hand, it underlies the pathogenesis of type 2 diabetes, atherosclerosis and hypertension - diseases that are currently the main causes of increased mortality.
The increasing prevalence of MS with age, taking into account the progressive aging of the population, dictates the need to find new methods and targets for the treatment of this potentially life-threatening condition. Due to the increasing proportion of polypharmacy and forced polytherapy in geriatric practice, it is advisable to concentrate efforts on finding predominantly non-drug methods for correcting the basic pathophysiological mechanisms of MS.
A.V. Krasilnikov, A.L. Azin
Republican Clinical Hospital of War Veterans, Yoshkar-Ola
Krasilnikov Alexey Vladimirovich – doctor of the therapeutic department
Literature:
1. Mamedov M.N. Metabolic syndrome is more than a combination of risk factors: principles of diagnosis and treatment. - M.: Vervag Pharma, 2006. - P. 7-42.
2. Mamedov M., Suslonova N., Lisenkova I. et al. Metabolic syndrome prevalence in Russia: Preliminary results of a cross-sectional population study. Diabetic and Vascular Disease research 2007; 4 (1); 46-47.
3. Zimmet P., Shaw J., Alberti G. Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabetic Medicine 2003; 20 (9): 693-702.
4. Tereshina E.V. Metabolic disorders - the basis of age-dependent diseases or aging of the body? State of the problem // Advances in gerontology, 2009. - T. 22. - No. 1. - P. 129-138.
5. Avogaro P., Crepaldi G., Enzi G., Tiengo A. Association of hyperlipidemia, diabetes mellitus amd mild obesity. Acta Diabetol Lat 1967; 4: 572-90.
6. Tiengo A., Avogaro P., Del Prato S. Pathogenesis and therapy of plurimetabolic syndrome. Nutr Metab Cardiovasc Dis 1996; 6: 187-192.
7. Haffner S. Insulin and blood pressure in the San Antonio heart study: a review. Cardiovasc Risk Factors 1993; 1: 18-27.
8. Tobey TA, Greenfield M., Kraemer F., Reaven GM Relationship between insulin resistance, insulin secretion, VLDL kinetics and plasma triglycerides levels in normotriglyceridemic men. Metabolism 1981; 30: 165-71.
9. Perova N.V., Metelskaya V.A., Oganov R.G. Pathogenetic basis of metabolic syndrome as a high-risk condition of atherosclerotic diseases. - International Medical Journal, 2001; 7 (3): 6-10.
10. Tiengo A., Avogaro P., Del Prato S. Pathogenesis and therapy of plurimetabolic syndrome // Nutr. Metab. Cardiovascular Disease, 1996. - Vol. 6. - P. 187-192.
11. Moiseev V.S., Ivleva A.Ya., Kobalava Zh.D. Hypertension, diabetes mellitus, atherosclerosis - clinical manifestations of metabolic syndrome X // Bulletin of Ross. Academy of Medicine Sciences, 1995. - No. 5. - P. 15-18.
12. Considine RV, Considine EL, Williams CJ et al. The hypothalamic leptin receptor in humans // Diabetes, 1996. - Vol. 45, N 7. - P. 992-994.
13. Larsson H., Elmstahl S., Ahren B. Plasma leptin levels correlate to islet function independently of body fat in postmenopausal women // Diabetes. - 1996. - Vol. 45, N 11. - P. 1580-1584.
14. Henriksen EJ, Jacob S, Kinnick TR et al. ACE inhibition and glucose transport in insulin-resistant muscle: roles of bradykinin and nitric oxide //Am J Physiol1. - 1999. - Vol. 277. - R332-R336.
15. Reaven GM Role of insulin resistance in human disease // Diabetes, 1988. - Vol. 37. - P. 1595-1607.
16. Mychka V.B., Gornostaev V.V., Shikina N.Yu., Chazova I.E. Arterial hypertension and obesity // Consilium medikum, 2001. - T. 3. - N 13.
17. Shestakova M.V. Diabetes mellitus in old age: clinical features, diagnosis and treatment // Consilium medikum, 2002. - T. 4. - N 10.
18. Angelo L. Gaffo N. Lawrence Edwards and Kenneth G. Saag. Gout. Hyperuricemia and cardiovascular disease: how strong is the evidence for a causal link? Arthritis Research & Therapy 2009, Vol. 11, Issue 4, pp. 240-9.
19. Hyon K., Choi Earl, S. Ford et al. Prevalence of the Metabolic Syndrome in Patients With Gout: The Third National Health and Nutrition Examination Survey Arthritis & Rheumatism 2007. Vol. 57, No. 1, R. 109-115.
20. 3. Teh-Ling Liou, Ming-Wei Lin, Li-Chuan Hsiao et al. Is Hyperuricemia Another Facet of the Metabolic Syndrome? J Chin Med Assoc 2006. Vol. 69, N 3, R. 104-109.
21. Barskova V.G., Nasonova V.A. Gout and insulin resistance syndrome. Russian Medical Journal, 2003. - No. 23, pp. 12-20.
22. RhoYH, Choi SJ, Lee YH et al. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci, 2005. - Vol. 20, Issue 6. R. 1029-33.
23. Juan García Puig, María Angeles Martínez. Hyperurecemia, Gout and the Metabolic Syndrome. Curr Opin Rheumatol, 2008. - Vol. 20, N 2, R. 187-191.
24. Sadulayeva I.A. Rhythm disturbances in metabolic syndrome. - Medical Bulletin, 2009. - No. 36 (505).
25. Korkushko O.V. Cardiovascular system and age: clinical and physiological aspects. - M.: Medicine, 1983. - 176 p.
How does metabolic syndrome manifest?
The presence of MS is indicated by a combination of several clinical symptoms:
- increased blood pressure;
- android type obesity (with predominant fat deposition in the abdominal and upper shoulder girdle);
- disruption of cholesterol metabolism;
- decreased tissue sensitivity to insulin.
A decrease in the sensitivity of cells and tissues of the body to the hormone insulin (insulin resistance) is one of the characteristic signs of metabolic syndrome. In this case, there is often a compensatory increase in this indicator in the blood to maintain blood sugar levels within target values. However, the body's reserve capabilities are individual and not unlimited, and ultimately this can lead to additional disruptions in functioning. As a result, various disorders arise - metabolic, hormonal, cardiovascular, etc.
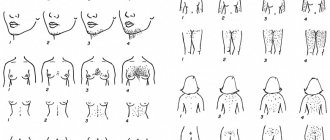
The pathological process develops slowly, often without pronounced symptoms. Frequent “companions” of excess body weight – high blood pressure, fatigue and shortness of breath – may indicate problems in the body. Visually, this endocrine disorder is signaled by a steadily expanding waistline: in patients with metabolic syndrome, excess fat accumulates mostly in the abdominal organs (which entails further disturbances in lipid and carbohydrate metabolism and complicates the very functioning of the organs).
You can check whether a person is at risk by measuring their waist circumference. With metabolic syndrome in women this parameter exceeds 80 cm, in men – 94 cm.
Other symptoms of metabolic syndrome
You can suspect that a person has metabolic syndrome if the following signs are present:
- increased fatigue, weakness, drowsiness;
- apathy;
- dyspnea;
- complaints of snoring and holding your breath during sleep;
- increased appetite;
- nocturia (nighttime urge to urinate);
- dry or sweaty skin, sometimes hyperkeratosis;
- unquenchable thirst.
Tips for Improving Metabolic Flexibility
There are two main ways to improve metabolic flexibility
: First, reduce your total carbohydrate intake in favor of protein and fat, which have already been shown to speed up fat burning in healthy people. Second, increase the intensity of training (aerobic and interval), this speeds up fat burning in both lean and obese people.
For people who are overweight and lead a sedentary lifestyle, exercise is a catalyst for increasing metabolic flexibility even if a change in diet does not produce results in a short period of time.
These studies show that high-intensity interval training can enhance fat burning in both lean and obese people. Combining diet with exercise will help you achieve optimal adaptation to maintain a slim figure and health.
According to one study in obese people, doing aerobic exercise increases fat burning, while restricting carbohydrates and increasing the amount of fat in the diet alone does not achieve this. Scientists believe exercise is a catalyst for improving metabolic flexibility in obese people.
We recommend
“The best courses in nutritionology abroad and in Russia” Read more
Before going to bed, sometimes consume “healthy” fat if you have difficulty falling asleep (tormented by hunger). If your blood sugar is low and your fat burning rate is low during sleep, then consuming a healthy fat like coconut oil may help. This approach helps avoid insulin spikes and deterioration in leptin production. In addition, the body's ability to burn fat at night increases.
Of course, keep clean breaks between meals (no snacking). Limiting your eating to 8-12 hours a day can increase fat burning and lead to weight loss. This method involves abstaining from food outside the “eating window”, which improves fat burning.
Diagnosis of metabolic syndrome
Only an endocrinologist can timely identify this syndrome and differentiate it from other pathologies with similar symptoms (for example, from hypothyroidism, which is often also manifested by weight gain) with the help of professional knowledge, as well as instrumental and laboratory studies (diagnosis of carbohydrate metabolism).
A consultation with an endocrinologist will include a study of the patient’s medical history, collection and analysis of anamnesis, a detailed physical examination with measurement of certain parameters (height, weight, waist and hip circumference, calculation of body mass index, etc.), measurement of blood pressure.
Laboratory diagnosis of metabolic syndrome includes assessment of the state of carbohydrate metabolism, lipid spectrum indicators, if necessary, this is supplemented by a number of hormonal studies.
Instrumental diagnostics of metabolic disorders may include 24-hour ECG monitoring, ABPM, polysomnography and other methods, including ultrasound, CT and MRI.
Also, the attending physician can refer the patient for consultation to other specialists - a cardiologist, gynecologist, andrologist, etc.
Diagnostics
Laboratory blood tests play an important role in diagnosis. The diagnosis is most often made by an endocrinologist, but the help of a cardiologist, nutritionist, or therapist may be needed.
The examination complex is complemented by:
- daily blood pressure monitoring;
- duplex scanning of the brachiocephalic arteries;
- various types of electrocardiogram;
- Ultrasound of the heart and blood vessels;
- cardiac rhythmography, cardiac screening, etc.
Metabolic syndrome. Treatment
Treatment of MS should be competent and comprehensive. Here it is important to take into account and try to normalize all metabolic disorders.
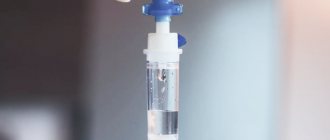
Very often, patients believe that metabolic syndrome requires immediate treatment with drugs. However, the first thing that needs to be done is to remove intermediate metabolic products and excess biologically active substances from the body. This is possible with the help of a course of membrane (filtration) plasmapheresis.
A course of plasmapheresis for metabolic syndrome is prescribed to normalize microcirculation and blood rheology, remove immune and fibrin monomer complexes, medium molecular toxins, and erythropoiesis inhibitors from it; stimulation of fibrinolysis, antithrombin activity; normalization of the permeability of the basement membrane of the glomeruli of the kidneys and, thereby, to restore various metabolic disorders.
Only then can the attending endocrinologist prescribe medications that help normalize metabolic processes and eliminate the symptoms of emerging disorders.
At the MedicCity multidisciplinary clinic, you can undergo diagnosis and treatment of metabolic syndrome and other endocrine diseases at a convenient time from high-class specialists. We will take care of your health!