What does Nimesulide help with and indications for use?
Before using the drug, consult a doctor.
It is important to remember that Nimesulide does not affect the causes of diseases and does not treat them. This drug is symptomatic and is used to relieve and alleviate clinical manifestations.
The main indications for taking the drug are:
- Diseases of the musculoskeletal system with severe pain (arthritis of various origins, including rheumatoid, bursitis, tendonitis, osteoarthritis, myalgia).
- Early postoperative period.
- Headache or toothache.
- Periodic pain in women.
Nimesulide (Nefopamum)
Undesirable side effects can be minimized by using the drug in the minimum effective dose with the minimum duration of use necessary to relieve pain.
There is evidence of very rare cases of serious reactions from the liver, including cases of death, associated with the use of nimesulide-containing drugs. If symptoms similar to signs of liver damage appear (anorexia, itching, yellowing of the skin, nausea, vomiting, abdominal pain, dark urine, increased activity of liver transaminases), you should immediately stop using nimesulide and consult a doctor. Repeated use of nimesulide in such patients is contraindicated. After 2 weeks of using the drug, monitoring of liver function parameters (“transaminases”) is necessary.
Liver reactions, which are in most cases reversible, have been reported with short-term use of the drug. While using nimesulide, the patient should refrain from taking other analgesics, including NSAIDs (including selective COX-2 inhibitors).
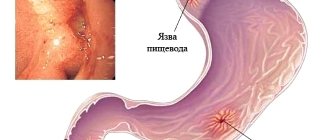
Nimesulide should be used with caution in patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), since exacerbation of these diseases is possible.
The risk of gastrointestinal bleeding, peptic ulcer/perforation of the stomach or duodenum increases in patients with a history of gastrointestinal ulceration (ulcerative colitis, Crohn's disease), as well as in elderly patients, with an increase in the dose of NSAIDs, so treatment should begin with the lowest possible dose. In such patients, as well as in patients who require the simultaneous use of low doses of acetylsalicylic acid or other drugs that increase the risk of gastrointestinal complications, it is recommended to additionally prescribe gastroprotectors (misoprostol or proton pump blockers). Patients with a history of gastrointestinal disease, especially older patients, should report new gastrointestinal symptoms (especially symptoms that may indicate possible gastrointestinal bleeding) to their physician.
Nimesulide should be prescribed with caution to patients taking drugs that increase the risk of bleeding (oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors or antiplatelet agents such as acetylsalicylic acid). If gastrointestinal bleeding or gastrointestinal ulceration occurs in patients taking nimesulide, treatment with the drug must be stopped immediately.
Given reports of visual disturbances in patients taking other NSAIDs, if any visual disturbance occurs, the use of nimesulide should be immediately discontinued and an ophthalmological examination performed.
The drug may cause fluid retention in tissues, so patients with arterial hypertension, renal and/or heart failure, coronary heart disease, peripheral arterial disease and/or cerebrovascular diseases, with risk factors for developing cardiovascular diseases (for example, hyperlipidemia, diabetes mellitus) diabetes, smokers) nimesulide should be used with extreme caution. If the condition worsens, treatment with nimesulide should be discontinued.
Clinical studies and epidemiological data suggest that NSAIDs, especially in high doses and with long-term use, may lead to a slight increase in the risk of myocardial infarction or stroke. There is insufficient data to exclude the risk of such events when using nimesulide.
If signs of a “cold” or acute respiratory viral infection occur during the use of nimesulide, the drug should be discontinued. Nimesulide can change the properties of platelets, so caution must be exercised when using the drug in people with hemorrhagic diathesis, however, the drug will not replace the preventive effect of acetylsalicylic acid in cardiovascular diseases.
Elderly patients are particularly susceptible to adverse reactions to NSAIDs. including the risk of gastrointestinal bleeding and perforations that threaten the patient’s life, decreased function of the kidneys, liver and heart. When taking nimesulide for this category of patients, proper clinical monitoring is necessary.
There is evidence of the occurrence in rare cases of severe skin reactions (such as exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis) when taking NSAIDs, including nimesulide. At the first manifestations of a skin rash, damage to the mucous membranes or other signs of an allergic reaction, nimesulide should be stopped immediately. The use of the drug may adversely affect female fertility and is not recommended for women planning pregnancy.
Contraindications
The drug is prohibited for gastric and duodenal ulcers, especially in the acute stage, for bleeding disorders and increased bleeding, Crohn's disease, bronchial asthma, inflammatory processes in the intestines, polyposis of the nasal cavity and paranasal sinuses.
Also, the use of Nimesulide is limited in case of individual intolerance to NSAIDs, severe chronic, renal and liver failure, in children under 12 years of age, during pregnancy and lactation.
Side effects and overdose
Taking Nimesulide in some cases may be accompanied by the development of unwanted side reactions. These include:
- From the side of the central nervous system: dizziness, anxiety, headaches, nightmares.
- Urinary system: decreased amount of urine excreted, hematuria, exacerbation of chronic renal failure.
- From the gastrointestinal tract: dyspeptic syndrome (nausea, vomiting, stool disorders), abdominal pain, flatulence, bleeding, gastritis, ulcerative lesions, jaundice as a result of cholestasis, increased transaminases.
- Skin: increased sweating and allergic reactions of varying severity (itching, urticaria, swelling, Stevens-Johnson syndrome).
- From the cardiovascular system: hypotension, increased heart rate.
- Hematopoietic organs: anemia, pancytopenia, increased clotting time.
- Respiratory system: shortness of breath, spasm of smooth muscles in the bronchi, exacerbation of COPD or bronchial asthma.
- Organ of vision: blurred perception.
Symptoms of overdose are similar to those of adverse reactions. They develop due to non-compliance with doctor’s instructions and uncontrolled use of Nimesulide.
In case of overdose, it is necessary to discontinue the drug, lavage the stomach to clean water and take activated carbon at the rate of 1 tablet per 10 kg of body weight.
Interactions
Nimesulide increases the activity of antiplatelet agents and anticoagulants. Concomitant use of this drug with diuretics reduces their effectiveness, which can lead to edema, especially in patients with hypertension and renal failure.
Nimesulide also increases the toxicity of cyclosporine and methotrexate. And its simultaneous use with GCS and serotonin reuptake inhibitors significantly increases the risk of bleeding in the digestive tract.
NIMESULIDE CANON
Interaction
Glucocorticosteroids:
Increase the risk of gastrointestinal ulcers or bleeding.
Antiplatelet agents and selective serotonin reuptake inhibitors:
increase the risk of gastrointestinal bleeding.
Anticoagulants:
NSAIDs may enhance the effect of anticoagulants such as warfarin. Due to the increased risk of bleeding, this combination is not recommended and is contraindicated in patients with severe coagulation disorders. If combination therapy cannot be avoided, careful monitoring of blood clotting parameters is necessary.
Diuretics:
NSAIDs may reduce the effect of diuretics. In healthy volunteers, nimesulide temporarily reduces the excretion of sodium under the influence of furosemide, to a lesser extent the excretion of potassium, and reduces the actual diuretic effect. Co-administration of nimesulide and furosemide leads to a decrease (by approximately 20%) in the area under the concentration-time curve (AUC) and a decrease in the cumulative excretion of furosemide without changing the renal clearance of furosemide. Co-administration of furosemide and nimesulide requires caution in patients with impaired renal or cardiac function.
ACE inhibitors and angiotensin II receptor antagonists:
NSAIDs may reduce the effect of antihypertensive drugs. In patients with mild to moderate renal failure (creatinine clearance 30-80 ml/min), when co-administered with ACE inhibitors, angiotensin II receptor antagonists or substances that suppress the cyclooxygenase system (NSAIDs, antiplatelet agents), further deterioration of renal function and the occurrence of acute renal failure, which is usually reversible. These interactions should be considered in patients taking nimesulide in combination with ACE inhibitors or angiotensin II receptor antagonists. Therefore, coadministration of these drugs should be used with caution, especially in elderly patients. Patients should be kept adequately hydrated and renal function should be closely monitored after initiating concomitant therapy.
There is evidence that NSAIDs reduce the clearance of lithium, which leads to increased plasma lithium concentrations and its toxicity. When prescribing nimesulide to patients receiving lithium therapy, plasma lithium concentrations should be regularly monitored. No clinically significant interactions were observed with glibenclamide, theophylline, digoxin, cimetidine and antacid drugs (for example, a combination of aluminum and magnesium hydroxides).
Nimesulide inhibits the activity of the CYP2C9 isoenzyme. When taking drugs metabolized with the participation of this isoenzyme with nimesulide, the concentration of these drugs in plasma may be exceeded,
When used simultaneously with antiepileptic drugs (valproic acid), antifungal drugs (ketoconazole), antituberculosis drugs (isoniazid), amiodarone, methotrexate, methyldopa, amoxicillin in combination with clavulanic acid, an additive hepatotoxic effect is possible.
Due to the high degree of binding of nimesulide to plasma proteins, patients concomitantly taking sulfonamides should be under medical supervision, undergoing examinations at short intervals. When prescribing nimesulide less than 24 hours before or after taking methotrexate, caution is required, since in such cases the concentration of methotrexate in plasma and, accordingly, the toxic effects of this drug may increase. Due to their effect on renal prostaglandins, inhibitors of prostaglandin synthetase, such as nimesulide, may increase the nephrotoxicity of cyclosporines.
In vitro studies
showed that nimesulide is displaced from the binding sites by tolbutamide and salicylic acid. Although these interactions were determined in blood plasma, these effects were not observed during clinical use of the drug.