Angina attacks, coronary heart disease and other pathologies are associated with insufficient oxygen supply to tissues. Trimetazidine tablets are used to prevent and treat these diseases. They strengthen the cells of the heart muscle (myocardium) and neurons, improve the condition of the brain and organ of vision.
Description of the drug "Trimetazidine"
"Trimetazadine" is a drug with the same active ingredient in the form of trimetazidine hydrochloride. Acts as a cardioprotective and antianginal agent. Release form: round tablets with a pink coating. One tablet may contain 20 or 35 mg of the active ingredient. Each package contains 30 or 60 pcs.
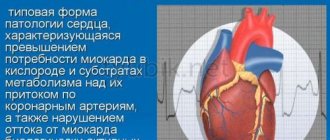
The use of tablets reduces tissue hypoxia and increases their oxygen saturation. This helps protect blood vessels and the heart muscle - the myocardium. Another positive effect is associated with improved condition of the brain, visual organs and vestibular system.
The active substance stimulates metabolism in the tissues of the myocardium and sensory organs, which is especially important in the presence of ischemia. Trimetazidine directly affects neurons - brain cells, as well as cardiomyocytes. As a result, oxygen consumption improves and chemical decarboxylation reactions are activated. This leads to increased energy efficiency of tissues, thereby protecting cells from damage.
The drug is also used in the presence of pathology of the organ of vision. Trimetazidine helps improve retinal function. The drug also reduces the number of attacks due to angina pectoris. Thanks to taking the medicine, it is possible to reduce changes in blood pressure in a relatively short time - after 10-15 days. Therefore, the ability to withstand physical and stressful loads increases.
Another positive result is the optimization of potassium concentration in myocardial cells, preventing the accumulation of sodium and calcium cations. This helps improve heart rate as the muscle begins to contract better.
The drug is sold only upon presentation of a prescription, its use is possible after the prior approval of a doctor. Tablets are stored in normal room conditions - temperature no more than 25 degrees, moderate air humidity, protected from light. Access for children is prohibited. The general shelf life is 3 years from the date of production.
Trimetazidine
Suction
After oral administration, trimetazidine is quickly and almost completely absorbed from the gastrointestinal tract. Bioavailability - 90%.
The time to reach the maximum concentration in the blood plasma is about 5 hours. Over 24 hours, the concentration of trimetazidine in the blood plasma is maintained at a level greater than and equal to 75% of the maximum concentration determined within 11 hours. The equilibrium concentration with regular use is achieved after 60 hours.
Concomitant food intake does not affect the bioavailability of trimetazidine.
Distribution
The volume of distribution is 4.8 l/kg. The degree of binding to plasma proteins is quite low - about 16% in vitro.
Removal
Trimetazidine is excreted from the body primarily by the kidneys (about 60% unchanged). The half-life in young healthy volunteers is about 7 hours, in patients over 65 years of age - about 12 hours.
The clearance of trimetazidine is characterized to a greater extent by renal clearance, which directly correlates with creatinine clearance (CC), and to a lesser extent by hepatic clearance, which decreases with patient age.
Special patient groups
Patients over 75 years of age
In patients over 75 years of age, increased plasma concentrations of trimetazidine may occur due to age-related decline in renal function.
A special study was conducted in a population of patients over 75 years of age while taking trimetazidine tablets 35 mg 2 times a day. An analysis performed by the kinetic population method showed an average twofold increase in plasma concentrations in patients with severe renal failure (creatinine clearance less than 30 ml/min) compared with patients with creatinine clearance more than 60 ml/min.
No safety differences were found in patients over 75 years of age compared with the general population.
Patients with kidney failure
The concentration of trimetazidine in blood plasma was increased on average by 2.4 times in patients with moderate renal failure (creatinine clearance 30-60 ml/min) and on average by 4 times in patients with severe renal failure (creatinine clearance less than 30 ml/min) compared to healthy volunteers with normal renal function.
No significant safety differences were observed in this patient population compared with the general population.
Use in children and adolescents
The pharmacokinetics of trimetazidine in children and adolescents under 18 years of age have not been studied.
Indications for the use of Trimetazidine tablets
The drug is indicated for use in the presence of the following indications:
- tinnitus;
- dizziness due to Meniere's disease;
- dizziness in case of vascular diseases;
- chorioretinal vascular disorders;
- ischemic heart pathology;
- angina attacks (used as a prophylactic agent).
Trimetazidine is also used in sports and bodybuilding. The drug strengthens the heart, which is especially important during regular intense exercise.
Therapeutic efficacy of trimetazidine in patients with coronary heart disease
Compounds that optimize oxygen utilization and the exchange of energy substrates in cardiomyocytes are called myocardial cytoprotectors, the most studied of which is trimetazidine. This drug, by selectively inhibiting mitochondrial 3-ketoacylCoA thiolase (3-KAT) and possibly carnitine palmitol transferase 1 (CPT-1), reduces fatty acid oxidation and stimulates glucose utilization. Under the influence of trimetazidine, under conditions of oxygen deficiency, the coupling of glycolysis and oxidative decarboxylation is restored, intracellular acidosis is reduced, and the amount of pyruvate transformed into acetyl-CoA increases, which ultimately leads to an increase in ATP production. Trimetazidine, by promoting the synthesis of more ATP molecules per consumed oxygen molecule, improves the balance between myocardial oxygen demand and supply. Elimination of the deficiency of intracellular ATP levels that occurs under conditions of myocardial ischemia forms the basis for the anti-ischemic cardioprotective effect of the drug. In addition, trimetazidine is actively involved in the utilization of stored fatty acids, stimulating the exchange of phospholipids in the sarcolemma. The consequence of this is a decrease in the content of free fatty acids and the creation of favorable conditions for restoring the structural integrity of cell membranes. An important result of the action of trimetazidine is the elimination of acidosis and high concentrations of intracellular calcium, characteristic of ischemia, hypoxia and overextension of cardiomyocytes observed in angina pectoris and chronic heart failure (CHF). The protective effect of trimetazidine against cardiomyocytes and endothelial cells reduces mechanical and endothelial dysfunction characteristic of ischemia and CHF. This provides protection of the myocardium from necrosis and apoptosis. Since the effects of trimetazidine are not associated with effects on hemodynamics, its use, unlike traditional antianginal drugs, does not increase the risk of arterial hypotension, bradycardia, conduction disorders and worsening CHF. Antianginal effectiveness of trimetazidine A large number of studies have been devoted to assessing the antianginal effectiveness of trimetazidine. P. Sellier noted that while taking the drug in 32 patients with coronary artery disease, exercise tolerance significantly increased and the ischemic threshold increased according to stress tests: volume of work performed (+25%, p = 0.012), duration of exercise (+114 s, p=0.016) and time until the appearance of ST segment depression 1 mm below the isoline (+90, p=0.034). J. Passeron in 54 patients with stable angina who received trimetazidine demonstrated a significant increase in the volume of work performed (+62%) and a significant decrease in the number of anginal attacks per week (–64%) in the absence of any changes in hemodynamic parameters or the development of significant side effects . According to a number of studies, by optimizing the metabolism of cardiomyocytes, trimetazidine not only has an antianginal effect, but also helps maintain the viability and functional activity of chronically ischemic myocardium. The positive effect of trimetazidine has been demonstrated in cases of impaired local myocardial contractility according to stress echocardiography with dobutamine. It is known that impaired local myocardial contractility is an earlier sign of myocardial ischemia than ECG changes. The results of echocardiography (increased left ventricular (LV) wall mobility index) indicate that, unlike placebo, trimetazidine significantly reduced myocardial ischemia during the pharmacological test. Moreover, while taking the drug, the infusion time (p = 0.019) and dobutamine dose (p = 0.003) significantly increased. This suggested that trimetazidine protects the myocardium from damage during ischemia. The results of double-blind controlled studies confirm the feasibility of using trimetazidine as the second component of combination therapy for stable angina. The impact on various parts of the pathogenesis of myocardial ischemia (myocardial oxygen demand, its delivery and utilization), on the one hand, is the physiological basis for the development of the additive effect of the components of such a combination, and on the other hand, it reduces the likelihood of developing side effects of therapy. A number of studies have demonstrated that combination therapy with trimetazidine and a b-blocker or calcium antagonist significantly improved the condition of patients with stable angina. In a multicenter, placebo-controlled study, 67 patients with stable angina who had failed previous treatment with diltiazem (180 mg/day) were randomized to receive additional trimetazidine (60 mg/day) or placebo. After 6 months Combination therapy in 87.5 (p<0.001) and 78% (p<0.01) of patients, respectively, reduced the number of angina attacks and nitroglycerin tablets taken by 25% in comparison with previous diltiazem monotherapy. The duration of exercise tests in both groups was similar, with the exception of the ischemic threshold, determined by the time of exercise before the appearance of ST segment depression 1 mm below the baseline. Thus, in the main group this indicator increased by 2 minutes. 41 s (p<0.001) and did not change significantly in the placebo group. In addition, the amount of work performed was also greater in the trimetazidine group (1445.9 kGm; p<0.001) than in the placebo group (563.7 kGm; p=0.012). In a study by A. Michaelides et al. in 53 patients with stable angina, the effectiveness of trimetazidine (60 mg/day) and isosorbide dinitrate (40 mg/day), prescribed in addition to propranolol (120 mg/day) when the previous therapy with the latter was insufficiently effective, was compared. It has been demonstrated that the combination of propranolol with trimetazidine, significantly more than the combination of propranolol with isosorbide dinitrate, reduces the number of angina attacks (by 63%; p<0.01 and 31%; p<0.01, respectively) and improves bicycle ergometry performance (time before the onset of ST segment depression by 1 mm increased by 81 s (p<0.05); the time before the development of a painful attack increased by 125 s, p<0.05 compared with monotherapy). Trimetazidine was well tolerated and had no effect on hemodynamic parameters. The results of this study showed greater effectiveness of the combination of trimetazidine with propranolol than two antianginal drugs with a hemodynamic mechanism of action. The ability of trimetazidine to enhance the antianginal effectiveness of b-blockers, calcium antagonists and nitrates, providing additional clinical effect and increasing exercise tolerance in patients with stable angina after 4 weeks. treatment, demonstrated in the TRIMPOL study. The TRIMPOL-2 study, which included 347 patients with stable angina resistant to metoprolol therapy (100 mg/day), confirmed not only the antianginal effectiveness of trimetazidine (60 mg/day) and the feasibility of its use as the second component of combination therapy , but also maintaining the positive effect of the drug on stress test performance after 3 months. treatment. In addition, a significant improvement in the course of the disease was also observed among patients with coronary artery disease who had previously undergone balloon coronary angioplasty or coronary artery bypass grafting. Similar results were obtained in the TACT (Trimetazidine in Angina Combination Therapy) study, which included 177 patients with stable angina who received monotherapy with long-acting nitrates (52%) or b-blockers (48%) for a week. Then, in addition to antianginal therapy, trimetazidine (90 patients) or placebo (n=87) was prescribed. After 12 weeks therapy, there was a decrease in the number of angina attacks from 5.6±0.6 to 2.7±0.5 in the trimetazidine group and in the placebo group - from 6.8±0.7 to 5.1±0.7 (p<0 .05), as well as the number of consumed short-acting nitrates - from 5.2±0.9 to 2.8±0.8 and from 5.5±0.8 to 4.1±0.9 (p>0.05 ) respectively. During trimetazidine therapy, a more significant increase in the duration of the stress test was observed (from 417.7±14.2 to 506.8±17.7 s in the main group and from 435.3±14.8 to 458.9±16.2 s in the placebo group (p<0.05)), time until the onset of ST segment depression by 1 mm (from 389.0±15.3 to 479.6±18.6 s in the main group and in the placebo group - from 411, 8±15.2 to 428.5±17.3 s (p<0.05)) and time before the onset of angina (from 417.0±16.9 to 517.3±21.0 s in the main group and in the placebo group – from 415.1±16.5 to 436.4±18.5 s (p<0.005)) with no effect on hemodynamic parameters. In the Russian TRIUMPH study, which included 846 patients with stable angina pectoris insufficiently controlled by hemodynamic antianginal drugs, 3-month therapy with trimetazidine (70 mg/day) in addition to combined traditional antianginal therapy, along with a decrease in the number of angina attacks and the consumption of short-acting nitrates in week (more than 3 times) significantly improved patients’ assessment of quality of life. These data confirm that trimetazidine can be prescribed at any stage of the treatment of stable angina to enhance the antianginal effectiveness of b-blockers, calcium antagonists and nitrates. M. Marzilli et al. A review of 23 large randomized, double-blind studies (1378 patients with stable angina) that compared the effectiveness of trimetazidine with placebo or other antianginal drugs demonstrated greater effectiveness of trimetazidine compared with placebo in terms of the effect on the clinical condition: number of angina attacks per week (average – 1.44, 95% CI –2.10 to –0.79; p<0.0001) and number of nitroglycerin tablets consumed per week (–1.47 95% CI –2.20 to –0.73 ; p<0.0001) and exercise tolerance – an increase in the time until the onset of ST segment depression by 1 mm (p=0.0002). Trimetazidine is effective in the treatment of stable angina, both as monotherapy and in combination with drugs that have a hemodynamic mechanism of action. In combination with traditional therapy for coronary artery disease, trimetazidine demonstrates an additive effect, which provides additional benefits to patients while maintaining the safety of treatment. Use of trimetazidine in patients undergoing coronary angioplasty A Greek study analyzed the effectiveness of trimetazidine in patients before and after percutaneous coronary intervention (PCI). 52 patients hospitalized with acute coronary syndrome were randomized to receive trimetazidine (60 mg/day) or placebo in addition to conventional antianginal therapy 15 days before PCI and for 3 months. after the procedure. Trimetazidine has been demonstrated to minimize myocardial reperfusion injury during PCI and improve global and regional contractility at 1 and 3 months. after operation. The use of trimetazidine in the treatment of coronary artery disease in patients with diabetes mellitus (DM) Higher morbidity and mortality among patients with diabetes compared to persons without this disease is associated with disorders of myocardial energy metabolism. Even in the absence of ischemia, cardiomyocytes of patients with diabetes absorb less glucose and lactate. The absolutely dominant role as a source of energy in this category of patients is played by beta-oxidation of fatty acids. Treatment of patients with diabetes and coronary artery disease should be aimed at reducing the intensity of beta-oxidation of fatty acids and restoring the coupling between glycolysis and oxidative decarboxylation of pyruvate. In this regard, the effectiveness of trimetazidine in patients with coronary artery disease and diabetes is of interest. In the open-label multicenter study TRIMPOL-1 (Trimetazidine in Poland), the efficacy and tolerability of a combination of trimetazidine and an antianginal drug was assessed in 50 patients with stable angina and diabetes. While taking trimetazidine (60 mg/day) for 4 weeks. clinical indicators significantly improved (the frequency of angina attacks was 3.06 versus 4.79 per week; p<0.01; the need for nitroglycerin was 2.29 versus 4.2 doses/week) and the parameters of the test with dosed physical activity: total work performed (9.39 vs. 8.67 MET, p<0.01), load duration (440.2 vs. 383.2 s; p<0.01), maximum ST segment depression (1.82 vs. 1, 91 mm), load time before the appearance of ST segment depression 1 mm below the isoline (358.3 vs. 301.6 s; p<0.01) and development of an anginal attack (400.0 vs. 238.3 s; p<0.01) 01) in comparison with the original data. The drug was well tolerated. These data confirm that in patients with coronary artery disease in combination with diabetes, trimetazidine can be an effective and safe addition to a drug with a hemodynamic mechanism of action. Fragasso, Rosano et al. demonstrated an improvement in the general condition and ejection fraction (EF) of the left ventricle (LV) in ischemic cardiomyopathy in patients with diabetes while taking trimetazidine. The use of trimetazidine in the treatment of coronary artery disease in elderly patients As patients age, the number of concomitant diseases increases, and the simultaneous administration of several drugs increases the risk of negative drug interactions. Moreover, there is evidence that older patients are more likely to develop side effects when taking antianginal drugs. By optimizing energy metabolism in the myocardium, cytoprotective drugs do not have a negative inotropic or chronotropic effect. Due to these properties, drugs of this class can be especially effective in the treatment of coronary artery disease in elderly patients (over 65 years of age). Thus, in the TRIMPOL-1 study, 71 patients over 65 years of age received trimetazidine for 4 weeks. significantly improved clinical indicators and parameters of stress tests: duration of exercise (p <0.01); load time until ST segment depression appears 1 mm below the isoline (50 s), load time before an angina attack occurs (126 s). Side effects were noted in 3% of patients. The TRIMEP (Trimetazidine in Elderly People) study, which included patients over 65 years of age, of whom 20% were over 70 years of age, noted the effectiveness of the combination of trimetazidine with traditional therapy and an increase in the quality of life of the subjects during treatment with this drug. The use of trimetazidine in the treatment of coronary artery disease in patients with metabolic syndrome (MS) Leonardo et al. in a randomized, double-blind, crossover study of 16 patients with MS treated with trimetazidine (60 mg/day) or atenolol (100 mg/day) over two 2-week periods, demonstrated beneficial effects of atenolol on clinical status, exercise capacity, and diastolic LV function and the absence of significant changes in these parameters during trimetazidine therapy. The authors suggested that the data obtained may be a consequence of various mechanisms responsible for the development of clinical symptoms in MS. A Polish study examined the effectiveness of 6-month therapy with trimetazidine in 34 patients with MS. Clinical status and exercise tolerance were assessed after 1 and 6 months. Negative results of the treadmill test were observed in 4 (11.76%) and 5 patients (14.71%) after 1 and 6 months. therapy with trimetazidine, respectively. A decrease in the number of angina attacks was demonstrated after 6 months. therapy (angina attacks were recorded in 76.47% before the start of therapy and in 38.23% after 6 months of treatment). There was no effect of trimetazidine on blood pressure and heart rate. The duration of the load increased significantly after 1 month. (652.9±206.2 vs. 563.4±190.4 s, p=0.0047) and 6 months. (650.3±207.8 s, p=0.0094) therapy. This data allowed the authors to conclude that trimetazidine therapy improves the clinical course of the disease and increases exercise tolerance in patients with MS. The use of trimetazidine in the treatment of CHF Treatment of CHF is one of the pressing problems of modern medicine due to the steady increase in morbidity, disability and mortality. The basis of pharmacotherapy for CHF is ACE inhibitors (ACEIs), diuretics, b-blockers. In most cases, the rational use of a combination of drugs with different mechanisms of action improves the effectiveness and quality of life of patients with CHF. However, the use of only drugs from these groups does not completely solve the problem of drug therapy for CHF, therefore, at present, not only existing approaches are being improved, but also new directions for the treatment of CHF are being developed. To date, a body of evidence has accumulated that trimetazidine, when added to hemodynamic therapy, has a beneficial effect on LVEF in patients with ischemic cardiomyopathy and LV systolic dysfunction. The effect of long-term therapy with trimetazidine on LV contractility in patients with ischemic cardiomyopathy was assessed by D. Napoli et al. Patients (61 people) with ischemic cardiomyopathy and XSN II - IV FC according to NYHA with systolic dysfunction (FV LV 30 ± 1%) were randomized for a double blind placbo -controlled study. 30 patients in addition to traditional therapy were prescribed trimetasidine (60 mg/day). After 18 months. treatment was noted by a decrease in FC CHN according to NYHA, an improvement in the contractility of the LV myocardium (FV LV was 7%, p <0.001, while in the comparison group this indicator decreased by 5%, p> 0.05), a slowdown in the LV remodeling process (of course- The diastolic volume of LV in the main group has slightly decreased, while in the comparison group it increased (p <0.001)). This was consistent with the stable level of C - reactive protein in blood plasma (inflammation marker) in the trimetasidine group and an increase in this indicator in the comparison group (the difference between the groups turned out to be reliable - p <0.001). The authors suggested that suppression of inflammation helps to improve metabolism in cardiomyocytes and endothelial cells, which leads to an increase in contractility and coronary blood flow, respectively, prevents cell damage and, ultimately, reduces tissue reaction and inflammation. In the placebo -controlled study, the effect of trimetasidine on the contractile function of the myocardium was studied in 38 patients with LV postinfarct dysfunction (LV FV on average 33%), randomized for the intake of trimetasidine or placebo. Before starting and after 2 months. Treatment to all patients performed stress - echocardiography with the introduction of a small dose of probutamine and a load test. In patients who received trimtazidin, a significant decrease in the index of segmental contractility was observed at rest and under load (p <0.001) and an increase in FV (p <0.001) without changing heart rate and blood pressure. In addition, in patients who received trimetasidine, compared with the placebo group, a reliable increase in oxygen volume at the load (p = 0.001) was noted. Vitale et al. It was shown that the addition of trimetasidine to traditional therapy improves the systolic and diastolic functions of LV, and also improves the quality of life of elderly patients suffering from ischemic cardiomyopathy. In a double blind, a placebo -controlled study, which included 20 patients with XN III and IV functional classes (FC) according to NYHA, the advantages of adding trimetasidine (60 mg/day) were studied to the classical treatment regimen of heart failure. After 6 months Patients who received trimetasidine observed a significant improvement in the state in terms of the severity of shortness of breath, the size of the FV LV and the volume of cardiac output (respectively p <0.001, p <0.018 and p <0.034) in comparison with the placebo group. In another double, blind cross -study with the participation of 15 patients with coronary heart disease and moderately reduced FV LV for 2 weeks. Trimetazidin (60 mg/day) or placebo was prescribed. The criteria for assessing the effectiveness of therapy were: increasing the stability of myocardium to ischemia, determined by the dose of probutamine and the time of its infusion during stress echocardiography, and improving the function of the LV on the index of segmental contractility at rest and at the peak of prebutamine. The duration of the study was 30 days. In the Trimetazidin group, the index of violation of segmental contractility both at rest and at the peak of the administration of probutamin significantly decreased (p = 0.013 and p = 0.018, ) and the introduced dose of dobutamin (from 22.7 to 28.7 mcg/kg/min., P = 0.003). Trimetazidin not only protected the myocardium from ischemic damage during the load, but also improved the local contractility of the LV myocardium at rest (this is due to a decrease in the number of myocardial giberica areas), while it did not affect the indicators of hemodynamics and consumption of oxygen myocardium. V.S. Zadionchenko et al. They studied the role of trimetasidine (predicting, “Gideon Richter”, Hungary) as part of the complex therapy of patients with heart failure. 60 patients with heart failure II - III FC (NYHA) with FV LV <45%were examined. All studied were distributed into 2 groups comparable in basic therapy, the severity of blood circulation insufficiency: the 1st group amounted to 40 patients on therapy of IAC, diuretics and B - B - 2 groups included 20 patients additionally received predictive. The dose of predisin was 35 mg 2 times/day, the duration of treatment - 5 weeks. In the course of the study, the clinical effectiveness of complex therapy, the quality of life of patients, the dynamics of the state of microcirculation, and the morphofunctional parameters of the heart were evaluated. Against the background of complex therapy with the addition of a predisin, the improvement of FC CHN was noted in 78% of cases, the clinical effectiveness of therapy was good; In 18% - satisfactory (however, FC CHF has not undergone changes), unsatisfactory - in 4% of cases. In the control group, the results of clinical efficiency were good in 66% of patients, satisfactory - in 24% and unsatisfactory - in 10%. The improvement (p <0.05) of the quality indicators under the influence of both methods of treatment, but significantly more pronounced - when the pre -zine therapy is included. Thus, under the influence of traditional therapy, there is a significant improvement in the quality of life (KZh) in the 1st group of patients, a decrease in the average total of the KZh from 69.1 ± 6.0 to 55.8 ± 5.2 (p <0.05) (p <0.05) (p <0.05) (p <0.05) (p <0.05) According to the Minnesotsky questionnaire, a decrease in the total score of KZh means improving the quality of life). Additional inclusion in the traditional healing complex of pre -zine was accompanied by more pronounced positive shifts in the indicators of the KH in the 2nd group of patients, a reliable (p <0.05) reduction of the average total of KZh from 69.1 ± 6.0 to 43.7 ± 5 , 0 (Table 1). The morphofunctional parameters of the heart have more improved in patients of the 2nd group: FV amounted to 41.6 ± 2.5 and 50.9 ± 3.3% to and after 5 weeks. treatment, respectively (p <0.05). CSR (final systolic volume) and KDO (final diastolic volume) decreased by 23.9 and 9.3%, respectively. In patients of the 1st Group, FV amounted to 40.7 ± 2.4 and 48.0 ± 2.2% to and at the end of treatment (p <0.05), CSO and KDO decreased by 20.6 and 9.5% respectively (Table 2). Combined therapy with the addition of pre -zine led to a reliable increase in FV LV and a decrease in the volume of the heart, which indicates an improvement in the contractile ability of the myocardium. Against the background of treatment in all patients, the average microcirculation (PM) has significantly increased. In patients who received the traditional therapy of heart failure, the average PM increased by 7.3%. However, the most pronounced PM increase occurred in a group that received a prediction - by 28.2% (p <0.05). In the 2nd group, intravascular resistance has significantly decreased - by 13.9% (p <0.05), in the 1st - by 11.2% (p <0.05). The decrease in endothelial activity in therapy with pre -zine is noteworthy - by 24.3%, while without it - by 15.3%. When distributing patients with heart failure according to hemodynamic types of microcirculation (GNM), an old one, which amounted to an average of 36%in groups, and spastic - 32%initially prevailed. The share of hyperemic and stagnant GTM accounted for 12 and 10%, respectively. The normocyriction MIM was detected in 10% of the examined patients of CHF. Against the background of therapy, severe forms of impaired microhemodynamics partially regressed. In the control group, the number of patients with a stasic GTM has not undergone changes, however, in the treatment of predisin, the incidence of the stasial type of GTM decreased by 8%. The number of patients with a spastic type decreased by 8% in the 1st group and by 12% in the 2nd, with stagnant GTM - by 8%, and it completely disappeared against the background of treatment with pre -zizin. The distribution of hyperemic GTM on average in groups remains constant. Against the background of treatment in both groups, the number of patients with the normocirculatory GTM increased (by 20–25%), which convincingly indicates an improvement in the state of microcirculation, which occurs due to a decrease in bloodstore in the peripheral vessels, and by normalizing its own smooth muscle activity of microsyudes. The positive dynamics of all indicators of microcirculation was more pronounced against the background of the use of pre -zine. The conclusion in all studies has demonstrated good tolerance of trimetazidine therapy. Side effects of the drug rarely developed and did not require the abolition of therapy. So, in the study of triumph of trimtazidine therapy, it was evaluated as excellent and good and doctors (98.4%) and patients (97.6%). Thus, the research results confirm that trimetasidine, improving cellular energy metabolism, reduces the severity and duration of ischemia periods, increases the contractility of the “sleeping” myocardium and improves the systolic and diastolic function of LV. The drug is able to enhance the therapeutic effect of traditional antianginal drugs, without affecting hemodynamic parameters. The results obtained convincingly indicate that the addition of trimetasidine to the traditional therapy of patients with II - III FC improves the clinical condition of patients, the contractile function of the myocardial, reduces microcirculation disorders to a greater extent than when using a complex of drugs that does not include myocardial cytoprotectors. All this allows you to name trimetasidine (predicting, Gideon Richter, Hungary) a promising drug and recommend it to this category of patients.
Literature 1. Branko V.V., Vakhlyaev V.D., Kamshilina L.S., Makolkin V.I. The influence of angiotensin-converting enzyme inhibitors on the state of microcirculation in patients with chronic heart failure // Cardiology. 1999. T. 39. No. 3. 2. Buziashvili Yu.I., Makolkin V.I., Osadchiy K.K., Asymbekova E.U., Akhmedyarova N.K., Starovoitova S.P. The effect of trimetazidine on reversible myocardial dysfunction in coronary heart disease // Cardiology. 1999. T. 39. No. 6. 3. Zadionchenko V.S., Shekhyan G.G., Yalymov A.A., Zasedateleva L.V. Microcirculation and morphofunctional status of patients with chronic heart failure during treatment with trimetazidine // Cardiovascular therapy and prevention. 2004. T. 3. No. 5. P. 74–80. 4. Sarkisov K.G., Dufak G.V. Laser Doppler flowmetry as a method for assessing the state of blood flow in microvessels // Methodology of flowmetry. 1999. pp. 9–14. 5. Tereshchenko S.N., Akimova O.S., Demidova I.V., Borisova N.E., Moiseev V.S. Cytoprotector trimetazidine in complex therapy of severe post-infarction chronic heart failure // Cardiology. 1999.T. 39. No. 9. 6. Makolkin V.I., Glezer M.G., Zharova E.A., Kapelko V.I., Karpov Yu.A., Lankin V.Z., Syrkin A.L. Myocardial ischemia: from understanding the mechanisms to adequate treatment // Cardiology. 2009. T. 40. No. 9. 7. Oganov R.G., Glezer M.G. Cardiology. 2007. No. 3. P. 4–13. 8. Belardinelli R., Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischemic cardiomyopathy // Eur. Heart J. 2001. Vol. 22. P. 2164–2170. 9. Colantuoni A., Bertuglia S., Integlietta M. Microvascular vasomotion: origin of laser doppler flux motion // Int. Microcirc.Clin. Exp. 1994. Vol. 23. R. 151–158. 10. Dalla-Volta S., Maraglino G., Della-Valentina P. et al. Comparison of trimetazidine with nife–dipine in effort angina. A double–blind crossover study // Cardiovasc. Drugs Therap. 1990. Vol. 4. P. 853–860. 11. Detry JM, Leclerca PJ on behalf of the TEMS Steering Committee. Trimetazidine European Multicenter Study versus Propranolol in stable angina pectoris: combination of Holter electrocardiographic ambulatory monitoring // Am. J. Cardiol. 1995. Vol. 76. P. 8–11. 12. Fagrell B., Intaglietta M. Microcirculation: its significance in clinical and molecular medicine // J. Intern. Medicine. 1997. Vol. 241. N 5. R. 349–362. 13. Kantor PF, Lucien A., Kozak R., Lopaschuk GD The antianginal drug Trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl CoenzymeAthiolase // Circ. Res. 2000. Vol. 86. P. 580–588. 14. Manchanda SC, Krishnaswami S. Combination treatment with trimetazidine and diltiazem in stable angina pectoris // Heart. 1997. Vol. 78. R. 353–357. 15. Passeron J. Clinical efficacy of trimetazidine in stable angina pectoris // Press. Med. 1986. Vol. 15. P. 1775–1778. 16. Sellier P. Chronic effects of trimetazidine on ergometric parameters in effort angina // Cardio-vasc. Drugs Ther. 1990. Vol. 4. P. 822–823.
Contraindications and side effects
The medicine should not be taken if there are such contraindications:
- hypersensitivity to the active or auxiliary component;
- pregnancy period;
- breastfeeding period.
Side effects are rare. They are mainly associated with allergic reactions, including itching and skin rashes. Nausea, vomiting, and gastralgia may be observed much less frequently. In such cases, you should stop using the tablets and consult your doctor. A break or change of therapy is possible.
Important!
The use of the drug does not affect the reaction and concentration in any way, nor does it reduce performance. Therefore, even drivers, machine operators and other machinery operators can take the pills.
Trimetazidine mv 35 mg 60 pcs. extended-release film-coated tablets
pharmachologic effect
Antihypoxic agent.
Composition and release form Trimetazidine mv 35 mg 60 pcs. extended-release film-coated tablets
Composition per 1 tablet:
- Active substance: trimetazidine dihydrochloride - 35 mg;
- Excipients (core): hypromellose - 93.0 mg, microcrystalline cellulose - 178.9 mg, colloidal silicon dioxide - 1.55 mg, magnesium stearate - 1.55 mg;
- Excipients (shell): opadry II 85F240012 pink - 10.0 mg, incl. polyvinyl alcohol - 4.0 mg, macrogol-3350 - 2.438 mg, red iron oxide dye - 0.04 mg, yellow iron oxide dye - 0.022 mg, talc - 1.48 mg, titanium dioxide - 2.02 mg.
Extended-release film-coated tablets 35 mg.
10, 20, 30 tablets in a blister pack made of polyvinyl chloride film and printed varnished aluminum foil.
10, 20, 30, 40, 50, 60 or 100 tablets in polyethylene terephthalate jars.
One can or 1, 2, 3, 4, 5, 6, 10 blister packs along with instructions for use are placed in a cardboard package (pack).
Description of the dosage form
Round, biconvex tablets, film-coated from light pink to pink. On a cross section, the core is white or almost white.
Directions for use and doses
Inside, during meals.
Trimetazidine MB is prescribed 1 tablet 2 times a day (morning and evening).
Patients with impaired renal function
In patients with moderate renal impairment (creatinine clearance from 30 to 60 ml/min), the drug is prescribed 1 tablet per day with breakfast.
Elderly patients
In elderly patients, trimetazidine exposure may be increased due to age-related decline in renal function.
It is recommended to take 1 tablet 1 time per day.
The benefit of therapy should be assessed after 3 months; if there is no effect, therapy should be discontinued.
Pharmacodynamics
Has an antihypoxic effect.
By directly influencing cardiomyocytes and neurons of the brain, the drug optimizes their metabolism and function. The cytoprotective effect is due to an increase in energy potential, activation of oxidative decarboxylation and rationalization of oxygen consumption (increased glycolysis and blockade of fatty acid oxidation).
Trimetazidine maintains myocardial contractility and prevents a decrease in the intracellular content of adenosine triphosphoric acid (ATP) and phosphocreatinine. In conditions of acidosis, it normalizes the functioning of membrane ion channels, prevents the accumulation of calcium and sodium ions in cardiomyocytes, and normalizes the intracellular content of potassium ions.
Reduces intracellular acidosis and increased phosphate levels caused by myocardial ischemia and reperfusion. Prevents the damaging effects of free radicals, preserves the integrity of cell membranes, prevents activation of neutrophils in the ischemic zone, increases the duration of the electrical potential, reduces the release of creatine phosphokinase (CPK) from cells and the severity of ischemic damage to the myocardium.
Trimetazidine reduces the frequency of angina attacks, reduces the need for nitrates, after 2 weeks of use it increases exercise tolerance, and sharp fluctuations in blood pressure (BP) are reduced.
Pharmacokinetics
After taking the drug orally, trimetazidine is quickly and almost completely absorbed from the gastrointestinal tract. Bioavailability - 90%. Food intake does not affect the bioavailability of trimetazidine. The time to reach maximum concentration in blood plasma is 3-6 hours. A stable state is achieved after 60 hours. The volume of distribution is 4.8 l/kg. Communication with blood plasma proteins - 16%. Trimetazidine is excreted from the body mainly by the kidneys (about 60% unchanged). The half-life is 8.4 hours, in patients over 65 years of age - about 12 hours. The renal clearance of trimetazidine directly correlates with creatinine clearance (CC), hepatic clearance decreases with age. Easily penetrates histohematic barriers.
Indications for use Trimetazidine mv 35 mg 60 pcs. extended-release film-coated tablets
Coronary heart disease: prevention of attacks of stable angina (as part of complex therapy).
Contraindications
- hypersensitivity to any component of the drug;
- severe renal failure (creatinine clearance (CC) less than 30 ml/min);
- age under 18 years (efficacy and safety have not been established);
- pregnancy and breastfeeding;
- Parkinson's disease, symptoms of parkinsonism, tremor, restless legs syndrome and other associated movement disorders.
With caution: patients with severe liver failure (clinical data are limited), renal failure (creatinine clearance more than 30 ml/min.), age over 75 years.
Application of Trimetazidine mv 35 mg 60 pcs. extended-release film-coated tablets during pregnancy and lactation
There are no data on the use of Trimetazidine MB in pregnant women. Animal studies have not shown any direct or indirect reproductive toxicity. Reproductive toxicity studies have shown no effect of trimetazidine on reproductive function in rats of either sex. As a precautionary measure, it is not recommended to use Trimetazidine MB during pregnancy.
It is not known whether trimetazidine or its metabolites are excreted in breast milk. Risk to the newborn/child cannot be excluded. If it is necessary to use the drug Trimetazidine MB during lactation, the issue of stopping breastfeeding should be decided.
special instructions
Trimetazidine MB is not intended for the relief of angina attacks and is not indicated for the initial course of treatment of unstable angina or myocardial infarction in the prehospital stage or in the first days of hospitalization!
If an attack of angina occurs, treatment (drug therapy or revascularization) should be reviewed and adapted.
Trimetazidine MB may cause or worsen symptoms of parkinsonism (tremor, akinesia, increased tone), so patients should be regularly monitored, especially the elderly. In doubtful cases, patients should be referred to a neurologist for appropriate examination.
If movement disorders appear, such as symptoms of parkinsonism, restless legs syndrome, tremor, instability in the Romberg position and unsteadiness of gait, Trimetazidine MB should be permanently discontinued.
Such cases are rare and symptoms usually resolve after discontinuation of therapy: in most patients, within 4 months after discontinuation of the drug. If symptoms of parkinsonism persist more than 4 months after discontinuation of the drug, you should consult a neurologist.
Falls associated with unsteadiness in the Romberg position and unsteady gait or hypotension may occur, especially in patients taking antihypertensive drugs.
If you miss one or more doses of the drug, you cannot take a higher dose at the next dose!
Impact on the ability to drive vehicles and operate machinery
Caution should be exercised when driving vehicles and performing work that requires increased speed of physical and mental reactions (risk of dizziness and weakness).
Overdose
There is only limited information on trimetazidine overdose. In case of overdose, symptomatic therapy should be carried out.
Side effects Trimetazidine mv 35 mg 60 pcs. extended-release film-coated tablets
Adverse reactions, defined as adverse events at least possibly related to treatment with trimetazidine, are given in the following gradation: very often (≥1/10); often (≥1/100,
From the digestive system: Often - abdominal pain, diarrhea, dyspepsia, nausea, vomiting. Unspecified frequency - constipation.
From the cardiovascular system: Rarely - a feeling of palpitations, extrasystole, tachycardia, a marked decrease in blood pressure, orthostatic hypotension, which may be accompanied by general weakness, dizziness or loss of balance, especially when taking antihypertensive drugs, “flushes” of blood to the skin of the face.
From the central nervous system: Often - dizziness, headache. Unspecified frequency - symptoms of parkinsonism (tremor, akinesia, increased tone), instability in the Romberg position and “unsteadiness” of gait, restless legs syndrome, other associated motor disorders, usually reversible after cessation of therapy; sleep disorders (insomnia, drowsiness).
From the skin and subcutaneous tissue: Often - skin rash, itching, urticaria. Unspecified frequency - acute generalized exanthematous pustulosis, Quincke's edema.
Other: often - asthenia.
From the circulatory system: Unspecified frequency - agranulocytosis, thrombocytopenia, thrombocytopenic purpura.
From the liver and biliary tract: Unspecified frequency - hepatitis.
Drug interactions
No information available.