Alkalosis
(Late Latin alcali - alkali, from Arabic al-quali) - a form of acid-base imbalance, characterized by a shift in the ratio between acid anions and blood base cations towards an increase in cations.
Considering the causes of alkalosis in clinical practice, it is customary to distinguish between gas or respiratory alkalosis, caused by excessive elimination of carbon dioxide, and metabolic alkalosis, associated with a disturbance in the exchange of “non-volatile” acids or bases (loss of acids or accumulation of excess bases), as well as with excessive introduction of bases into the body (see Acids and Bases).
Both types of alkalosis, depending on the effect on the pH value of the blood, can be compensated or decompensated. Compensated alkalosis is understood as a violation of the acid-base balance in which the pH is within the physiological norm - 7.35-7.45 (see Acid-base balance). Normal pH values in these cases indicate that the ratio of the concentrations of the components of the carbonate buffer has changed slightly, in contrast to the absolute values of H2CO3 and NaHCO3, which can undergo more significant changes.
Along with fully compensated disturbances of acid-base balance, O'Sigaard-Andersen, R. Winters, K. Engel (O'Sigaard-Andersen, R. Winters, K. Engel) distinguishes a group of partially compensated conditions in which there is incomplete compensation of one of the components of the carbonate buffer systems.
Decompensated alkalosis is understood as a violation of the acid-base balance in which the pH shifts to the alkaline side (pH>7.45). This shift is usually due to a significant increase in base excess and depletion of physiological and physicochemical compensation mechanisms (see Acid-base balance, regulation).
1.General information
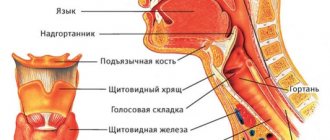
As is known, the human body maintains homeostasis - the constancy of a number of internal conditions, biochemical and biophysical parameters necessary to ensure normal life activity.
One of the most important is the pH value, or the “acid-base reaction pH” (pH), which is remembered from school chemistry lessons. A shift in this indicator in one direction or another grossly disrupts the functioning of all major organs and leads to the development of a serious, and if measures are not taken in a timely manner, a life-threatening condition. “Acidification”, i.e. An abnormal decrease in blood pH is called acidosis. Accordingly, alkalosis is a shift in pH to the alkaline side, i.e. excess pH level = 7.35 7.45.
A must read! Help with treatment and hospitalization!
Alkalosis is a violation of the acid-base balance in the body, characterized by an excess of bases (substances that have alkali properties) in the blood.
Bases (alkalis) are substances that are capable of attaching hydrogen ions. An indicator reflecting the acidity or alkalinity of solutions is pH. The higher the concentration of bases in the blood, the higher the pH level.
Normal blood pH ranges from 7.36 to 7.44. An increase in this indicator above 7.44 indicates alkalosis.
The occurrence of various biochemical processes and the operation of enzyme systems require the presence of certain constant conditions in the body. One of them is acid-base balance.
This parameter is maintained due to the work of the lungs, kidneys, and the activity of buffer systems in the blood (chemicals that help equalize the acid-base balance).
Depending on the causes of occurrence, alkalosis can be respiratory (respiratory) and metabolic (metabolic).
The leading mechanism for the development of respiratory alkalosis is an increase in pulmonary ventilation. Due to this, the concentration of carbon dioxide and carbon dioxide in the blood decreases, which causes a shift in blood pH towards the alkaline side.
The occurrence of metabolic alkalosis can be caused by large losses of acidic gastric contents (for example, with profuse and frequent vomiting), the use of certain diuretics, disruption of the adrenal glands (organs that synthesize hormones that affect water-salt and other types of metabolism), excessive intake of the body of substances with alkaline properties (for example, when correcting acidosis).
Treatment is primarily aimed at treating the underlying disease that caused the development of alkalosis. To correct the blood pH level, intravenous infusions of acidic solutions may be required.
In respiratory alkalosis, breathing with gas mixtures containing carbon dioxide is used, which helps normalize the acid-base balance.
Synonyms Russian
Metabolic alkalosis, respiratory alkalosis.
English synonyms
Alkalosis, Metabolic Alkalosis, Respiratory Alkalosis.
Symptoms
Symptoms are often masked by manifestations of the underlying disease, which caused the development of alkalosis.
Alkalosis may have the following symptoms:
- headache
- general weakness, lethargy
- dizziness
- disturbance of consciousness up to coma
- rave
- cramps in various muscle groups
- tetany (prolonged, strong convulsive muscle contractions)
- chest pain
- heart rhythm disorder
General information about the disease
Alkalosis is a violation of the acid-base balance in the body, characterized by an excess of alkalis in the blood.
Depending on the reasons that caused its development, alkalosis can be respiratory (respiratory) and metabolic (exchange).
Metabolic processes in the body occur with the consumption of oxygen and the formation of carbon dioxide. During breathing, excess carbon dioxide is removed through the lungs and the blood is enriched with oxygen.
For normal life, it is necessary to maintain certain concentrations of oxygen and carbon dioxide in the blood. Carbon dioxide, when combined with water, forms carbon dioxide, so its increased losses with increased and deepening of breathing can lead to the development of alkalosis.
With respiratory alkalosis, a decrease in the concentration of carbon dioxide leads to a narrowing of the blood vessels in the brain, which negatively affects its blood supply. This is associated with the appearance of symptoms such as dizziness, fainting, and impaired consciousness.
These and other conditions can lead to an increase in pulmonary ventilation and the development of respiratory alkalosis:
- severe pain from the nervous system
- excitement, anxiety
- psychosis
- increase in body temperature
- cerebrovascular accident (stroke)
- brain injury
- brain tumors
- from the lungs pneumonia
- bronchial asthma
- Chronical bronchitis
- pneumothorax (a collection of air in the chest that compresses the lung)
- other causes of sepsis – penetration of pathogenic microorganisms into the blood and spread of infection throughout the body
- heart failure
- liver failure
One of the causes of metabolic alkalosis is the loss of large amounts of gastric contents (for example, with frequent vomiting). Gastric juice is acidic due to the hydrochloric acid it contains, which is necessary for digesting food. As a result, the release of stomach contents can lead to metabolic alkalosis.
The use of certain diuretic drugs can cause the development of acalosis as a result of disturbances in the water and electrolyte balance in the body.
Metabolic alkalosis can occur during various pathological processes that lead to changes in the concentration of electrolytes in the body. For example, excessive formation of the hormone aldosterone causes the excretion of potassium and hydrogen ions in the urine, and the retention of sodium and water in the body. The release of hydrogen ions leads to an increase in blood pH (alkalosis).
Aldosterone is secreted by the adrenal glands (paired organs that secrete hormones necessary to regulate metabolism and water-electrolyte balance in the body). Increased secretion of aldosterone can be associated both with diseases of the adrenal glands (primary hyperaldosteronism) and with other pathological processes in which there is a decrease in the volume of fluid in the bloodstream (for example, with heart failure, cirrhosis of the liver).
At the same time, the resulting changes in water-electrolyte balance and blood pH lead to serious disruptions in the functioning of various organs and systems. For example, a decrease in potassium levels causes general weakness, muscle pain, and heart rhythm disturbances. Due to a decrease in calcium concentration, painful muscle cramps may occur before the development of attacks of tetany (strong and prolonged convulsive contractions, spasms of various muscles).
Treatment of alkalosis is conservative and consists of compensating the patient’s condition for the underlying disease, correcting the blood pH level and water and electrolyte disturbances by intravenous administration of special solutions.
In case of respiratory alkalosis, therapeutic measures are primarily aimed at normalizing the rhythm and depth of breathing.
Who is at risk?
Risk groups include:
- persons taking certain medications (eg, diuretics, aspirin)
- persons with damage to the nervous system (traumas, strokes, brain tumors)
- people who have lost large amounts of gastric contents (eg, excessive vomiting, aspiration of gastric contents with a tube)
- persons suffering from lung diseases (for example, pneumonia, bronchial asthma)
- persons with impaired functioning of the adrenal glands.
Diagnostics
Laboratory research methods are of key importance for the diagnosis and correction of alkalosis. Tests are carried out to determine the blood pH level, its gas composition, electrolyte concentrations and other vital parameters.
Laboratory research:
- Determination of blood pH. The determination of this parameter is based on identifying the concentration of hydrogen ions in arterial blood. With alkalosis, this parameter will be reduced and the pH level will be higher than 7.44.
- Determination of blood gas composition. Blood gas composition is determined using gas analyzers. These devices also allow you to set the blood pH. With alkalosis, the concentration of carbon dioxide in the blood will be reduced.
- General blood analysis. This analysis allows you to evaluate the main blood parameters: the number of red blood cells, hemoglobin, leukocytes, platelets, hematocrit number (the ratio of the number of blood cells to its liquid part). This is of great importance for establishing the causes of alkalosis. For example, one of the possible causes of respiratory alkalosis may be severe anemia, in which a decrease in hemoglobin, red blood cells and hematocrit number will be observed.
- General urine analysis with microscopy. This analysis allows us to identify the basic physical and chemical properties, establish the pH level of urine, and determine the presence of pathological and physiological metabolic products.
- Potassium, sodium, chlorine in serum. Potassium, sodium, chlorine are the main electrolytes in human blood, which are necessary for moving substances inside cells and removing metabolic products from them, maintaining acid-base balance, and carrying out other processes in the body. With alkalosis, significant changes in water and electrolyte balance can occur, which require constant monitoring and correction.
- Aldosterone. Aldosterone is an adrenal hormone that promotes the retention of sodium and water in the body and the excretion of potassium by the kidneys. One of the causes of metabolic alkalosis may be an increased level of this hormone.
- Cortisol. Cortisol is a hormone produced by the adrenal glands. It is involved in the regulation of the metabolism of proteins, fats and carbohydrates in the body, the formation of the body's adaptive response to stress. Determination of the level of this hormone may be required if Cushing's syndrome is suspected, in which alkalosis may occur.
- Alanine aminotransferase (ALT). An enzyme that is present in many cells of the body, but is found in greater quantities in liver cells. When liver cells are damaged, the level of this enzyme in the blood increases significantly. Liver failure may be one of the causes of alkalosis.
Research:
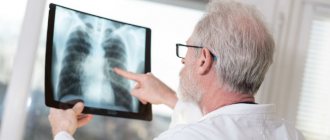
- Radiography. Chest x-rays can identify pathologies in the lungs that may be the cause of respiratory alkalosis (for example, pneumonia).
- Ultrasound examination (ultrasound). This study is based on the properties of ultrasound. The method makes it possible to visualize internal organs, identify changes in their structure, size, and allows you to diagnose the presence of space-occupying formations (for example, cysts, tumors), which is of great importance for identifying the causes of changes in the acid-base balance in the body.
- Computed tomography (CT) and magnetic resonance imaging (MRI). These studies have different operating principles. The computed tomography method is based on the ability of X-ray radiation to pass through tissues of varying densities. Images of human internal structures during MRI are formed as a result of the action of a strong magnetic field on the tissue, followed by registration and computer processing of the received signals.
These techniques make it possible to obtain highly informative layer-by-layer images of internal organs, which is very important in diagnosing the causes of various conditions, including alkalosis (for example, identifying tumors of the adrenal glands, brain).
Treatment
Treatment of alkalosis consists of treating the underlying disease or eliminating other causes that led to the development of this condition. With metabolic alkalosis, there is often a need to correct not only blood pH, but also disturbances in water and electrolyte balance. For this purpose, intravenous infusions of special solutions are performed.
To treat respiratory alkalosis, inhalation of gas mixtures containing carbon dioxide can be used.
Prevention
There is no specific prevention of alkalosis, since this condition can be a consequence of various pathological processes occurring in the body.
Recommended tests
- Blood pH determination
- Determination of blood gas composition
- General blood analysis
- General urine analysis with microscopy
- Potassium, sodium, chlorine in serum
- Aldosterone
- Cortisol
- Alanine aminotransferase (ALT).
2. Reasons
Depending on the specific causes of alkalosis, there are several main types.
Thus, gas (respiratory) alkalosis develops with hyperventilation - excessively intense breathing, when an abnormally high amount of oxygen enters the body. In turn, common causes of this condition include tumors and inflammatory lesions of the brain, intoxication with certain substances of organic and medicinal origin (in case of overdose), febrile temperature, and massive blood loss.
Among non-gas alkaloses, there are three main forms: excretory, exogenous and exchange (metabolic).
Thus, excretory alkalosis develops due to the loss of large volumes of substances with an acidic pH reaction, for example, gastric juice with uncontrollable vomiting, gastric fistulas, etc., as well as with some kidney diseases, hyperhidrosis (pathological sweating), prolonged use of diuretics .
Exogenous (due to external factors) alkalosis occurs, for example, when consuming large doses of baking soda to reduce high acidity in hyperacid forms of gastritis and peptic ulcers.
Metabolic alkalosis, by definition, occurs with etiologically different (hereditary or acquired) metabolic disorders, primarily electrolytes.
Finally, mixed type alkalosis is occasionally observed, in which respiratory and non-gas etiopathogenesis are combined (for example, in severe traumatic brain injuries, when hyperventilation is accompanied by intense vomiting).
Visit our Therapy page
Alkalosis
Symptoms of gas alkalosis reflect the main disorders caused by hypocapnia - hypertension of the cerebral arteries, hypotension of the peripheral veins with a secondary decrease in cardiac output and blood pressure, loss of cations and water in the urine. The earliest and leading signs are diffuse cerebral ischemia - patients are often excited, anxious, may complain of dizziness, paresthesia on the face and limbs, quickly get tired of contact with others, concentration and memory are weakened. In some cases, fainting occurs. The skin is pale, gray diffuse cyanosis is possible (with concomitant hypoxemia). Upon examination, the cause of gas alkalosis is usually determined - hyperventilation due to rapid breathing (up to 40-60 respiratory cycles per minute), for example: with thromboembolism of the pulmonary arteries; pulmonary pathology, hysterical shortness of breath (so-called dog breathing) or due to a mode of artificial ventilation of the lungs above 10 l/min. As a rule, there is tachycardia, sometimes a pendulum-like rhythm of heart sounds; pulse is small. Systolic and pulse blood pressure are slightly reduced when the patient is in a horizontal position; when he is transferred to a sitting position, orthostatic collapse is possible. Diuresis is increased. With prolonged and severe gas alkalosis (pCO2 less than 25 mm Hg), dehydration of the body and the appearance of seizures as a result of developing hypocalcemia may occur. In patients with organic pathology of the central nervous system and “epileptic readiness,” gas alkalosis can provoke an epileptic seizure. The EEG reveals an increase in amplitude and a decrease in the frequency of the main rhythm, bilateral synchronous discharges of slow waves. ECG often reveals diffuse changes in myocardial repolarization.
Metabolic alkalosis , which often appears with the use of mercury diuretics and with massive infusions of alkaline solutions or nitrate blood into the patient, is usually compensated, is transient in nature and does not have pronounced clinical manifestations (some respiratory depression and the appearance of swelling are possible). Decompensated metabolic alkalosis usually develops as a result of primary (with prolonged vomiting) or secondary (from potassium loss during massive hemolysis, diarrhea) loss of chlorine by the body, as well as in terminal conditions, especially accompanied by dehydration. Progressive weakness, fatigue, thirst are noted, anorexia, headache, and minor hyperkinesis of the muscles of the face and limbs appear. Convulsions due to hypocalcemia are possible. The skin is usually dry, tissue turgor is reduced (swelling is possible with excessive fluid infusion). Breathing is shallow, rare (unless pneumonia or heart failure is associated). As a rule, tachycardia, sometimes embryocardia, is detected. Patients first become apathetic, then lethargic, drowsy; subsequently, disorders of consciousness worsen until the development of coma. The ECG often reveals low T wave voltage and signs of hypokalemia. Hypochloremia, hypokalemia, and hypocalcemia are detected in the blood. The reaction of urine in most cases is alkaline (in case of A., due to primary losses of potassium, it is acidic).
Chronic metabolic alkalosis , which develops in patients with peptic ulcers due to long-term intake of large quantities of alkalis and milk, is known as Burnett's syndrome, or milk-alkali syndrome. It is manifested by general weakness, loss of appetite with aversion to dairy foods, nausea and vomiting, lethargy, apathy, itching, in severe cases - ataxia, deposition of calcium salts in tissues (often in the conjunctiva and cornea), as well as in the kidney tubules, which leads to the gradual development of renal failure.
3. Symptoms and diagnosis
The most common mechanism for the development of the clinical picture is circulatory disorders: blood pressure and blood supply to the heart and brain decrease. Muscle spasms and cramps, fainting, disturbances in intestinal motility, and a significant decrease in mental productivity may occur.
With gas alkalosis, changes in mental status can also be observed: agitation, excitement, etc.; Individuals with lesions of the central nervous system and a predisposition to epilepsy often develop epileptic seizures.
Non-gas types of alkalosis are characterized by severe weakness, increasing fatigue, headache, thirst with lack of appetite, sometimes convulsions or tremors; in chronic forms, skin itching, apathy, and pathological changes in the kidneys may develop.
The diagnosis is suggested clinically and confirmed laboratory.
About our clinic Chistye Prudy metro station Medintercom page!