What is pemphigus? Symptoms, causes and treatment
Pemphigus is a chronic autoimmune disease characterized by the appearance of a special type of blisters on the surface of previously healthy skin and mucous membranes. Among the types of pemphigus can be distinguished: vulgar, vegetative, erythematous and foliate.
Pemphigus can be diagnosed if acantholytic cells are detected, which are detected in a smear taken or as part of blisters in the epidermis itself (during histological examination). To treat pemphigus, glucocorticosteroids are first used (a whole course of treatment is prescribed). The latter always goes well with extracorporeal hemocorrection (plasmophoresis, cryoapherosis, hemosorption).
Causes
The reasons for the development of pemphigus have not yet been fully studied. One of the main causes of pemphigus is a violation of autoimmune processes, thereby the cells become antibodies to the immune system.
Violation of cell structure is subject to the influence of external factors, as well as aggressive environmental conditions. As a result, the communication between cells is disrupted, which leads to the formation of bubbles. The incidence rate in people with a hereditary predisposition is much higher.
Immunopathology of PSP
Humoral immunity
Because desmosomal cadherins are the only desmosomal components exposed on the cell surface, autoantibodies against desmosomal cadherins were first suspected to cause suprabasal acantholytic bullae in PSP. This was clearly confirmed by a study using newborn mice injected with IgG from PSP patient serum. In this study, mice treated with anti-desmoglein-depleted antibodies were protected from blisters, whereas anti-desmoglein3 antibodies caused acantholytic blisters.
However, some patients with PSP who have suprabasal mucosal acantholysis and skin blisters do not have circulating anti-desmoglein autoantibodies. This phenomenon is also observed in pemphigus, one of the autoimmune bullous mucosal diseases characterized by anti-desmoglein autoantibodies. In some cases demonstrating the pemphigus phenotype, the blisters may develop due to autoantibodies against desmoglein 3, but not against desmogleins. These data confirm that the mechanism of acantholysis in PSP varies among patients.
A recent study showed that antibodies to A2ML1, which act as a protease inhibitor, reduce the adhesion of cultured normal human keratinocytes by activating plasmin. This suggests that anti-A2ML1 autoantibodies from PSP patient serum may contribute to the induction of acantholysis. In addition, whether antibodies against the plakin family play a role in the induction of acantholytic blisters in PSP remains to be determined. Thus, further studies are needed to clarify the precise role of autoantibodies in the development of acantholytic blisters in PSP.
Bronchiolitis obliterans was first studied using bronchial biopsies from patients with PSP. In the bronchial epithelium, ciliated basal cells adhered to the lamina propria, whereas ciliated columnar cells became detached. In accordance with histological data, linear deposition of IgG was observed in the intercellular spaces of respiratory epithelial cells, as well as in the basement membrane area.
These data provided evidence that humoral immunity may contribute to the development of bronchiolitis obliterans in PSP. However, it is still unclear which types of autoantibodies are pathogenic in bronchiolitis obliterans.
It is important to note that desmosomal cadherins are differentially expressed between skin and bronchi. In particular, desmoglein 1 and 3, which are expressed in the skin epidermis and mucosa, are not expressed in normal respiratory epithelium. However, desmoglein 3 can be ectopically expressed in the lungs in cases of squamous metaplasia in response to inflammation. Thus, anti-desmoglein 3 antibodies may contribute to the development of bronchiolitis obliterans. In a recent study, mice treated with anti-epiplakin antibodies showed loss of intercellular adhesion in the respiratory epithelium. It has been suggested that anti-epiplakin antibodies may play a pathogenic role in the development of bronchiolitis obliterans, although epiplakin is located in the subcellular region of epithelial cells.
Human IgG is divided into four subclasses: IgG1, IgG2, IgG3 and IgG4. Among the anti-desmoglein antibody subclasses, the IgG1 subclass is dominant in the sera of patients with PSP, while antibodies of the IgG4 subclass are pathogenic in patients with pemphigus vulgaris and pemphigus foliaceus. In human immunity, IgG1 is the major isotype of Th1 immunity, whereas IgG4 is mainly secreted during the Th2 response.
Thus, the above results indicate that the Th1 response may be dominant in PSP. In addition, anti-desmoglein-3 antibodies from sera from PSP patients react with all five extracellular (EC) subregions of human desmoglein 3, whereas anti-desmoglein-3 antibodies from sera from pemphigus vulgaris patients primarily bind to the EC1 and EC2 domains. Pathogenic epitopes of desmoglein 3 also differ between PSP and pemphigus vulgaris. Pathogenic monoclonal antibodies in PSP bind to the EC2 and EC3 domains, in contrast to antibodies from pemphigus vulgaris, which bind to the EC1 domain. Differences in the distribution of desmoglein epitopes and subclasses reflect differences in the mechanisms mediating autoimmunity in PSP and pemphigus vulgaris.
Cellular immunity
The presence of lichenoid dermatitis in PSP indicates that cell-mediated immune mechanisms play a critical role in its development. Infiltration of CD8+ T cells and apoptotic keratinocytes are frequently observed in the epidermis of PSP, suggesting that the formation of lichenoid dermatitis is promoted by autoreactive CD8+ T cells targeting epidermal components. CD56+ cells are also found in lichenoid dermatitis, but further studies are needed to characterize these cells because CD56 is expressed on CD8+ T cells as well as natural killer cells.
Regarding CD4+ T-cell immunity, adoptive transfer of desmoglein-3-specific CD4+ T cells into RAG2 −/− mice was found to cause cell-mediated immune dermatitis, which resolved after administration of CD4 + T-suppressing interferon gamma -cells. Lichenoid dermatitis may be the only sign of PSP or may develop before blisters appear. Thus, this suggests that lichenoid inflammation caused by cell-mediated immunity may cause autoantigens such as plakins to be influenced by the immune system to produce autoantibodies.
In addition to mucosal lesions, marked infiltration of CD8 + T cells is observed in PSP-associated bronchiolitis obliterans and in the lungs of DSG3 −/− mice injected with IgG from the serum of PSP patients. These data point to CD8+ T cell immunity in the pathogenesis of bronchiolitis obliterans. In addition, adoptive transfer of desmoglein 3-specific CD4 + T cells into RAG2 −/− mice induced lung inflammation and ectopic expression of desmoglein 3. Therefore, both humoral and cellular immunity may be involved in the development of bronchiolitis obliterans in PSP, although for Understanding the precise pathophysiological mechanisms underlying bronchiolitis obliterans will require further research.
Potential pathomechanisms of paraneoplastic autoimmunity
Violation of central tolerance
T cells develop in the thymus and are subject to positive and negative selection during development before reaching the periphery. During positive selection, T cells that cannot interact with major histocompatibility complex molecules are removed from the thymic cortex. . Autoreactive T cells bearing T cell receptors with high affinity for self-peptide-bound MHC molecules are eliminated by negative selection in the thymic medulla.
In this process, tissue-specific antigens are expressed in the medullary epithelial cells of the thymus under the influence of factors such as the autoimmune regulator (AIRE). If the process of negative selection cannot be precisely controlled due to the presence of a tumor in the thymus, autoreactive T cells can escape central tolerance and promote autoimmunity in the peripheral region.
Thymoma is a neoplasm commonly associated with PSP. PSP patients with benign thymoma are usually cured after complete tumor resection.
It is well known that thymoma causes an autoimmune response. Indeed, other autoimmune diseases, including myasthenia gravis, can occur in patients with thymoma, and thymoma-associated PSP is often accompanied by myasthenia gravis. Thymoma has absent or reduced medullary parts and a defect in AIRE expression. T cells from AIRE − / − mice induced the production of antibodies against desmoglein 3 when interacting with DSG3 − / − B cells, and AIRE -dependent medullary thymic epithelial cells expressed desmogleins.
However, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, an inherited human disease with AIRE deficiency, does not contain anti-desmoglein or anti-BPAG1 antibodies, nor the clinical features of PSP. Recently, in a patient with an AIRE-expressing thymoma, the condition manifested as pemphigus with autoantibodies against desmoglein 1. These results suggest that AIRE may not be the only factor regulating central tolerance in PSP. Given that thymic factors other than AIRE (e.g., Fezf2) also contribute to negative selection, the mechanism of central tolerance breakdown in PSP should be further clarified.
Impaired peripheral tolerance
Even though thymic selection produces high-purity T cells that recognize foreign antigens, some self-reactive T cells migrate to the periphery. However, peripheral tolerance prevents the activation of autoreactive T cells in peripheral tissues through several mechanisms, including T cell anergy and deletion and suppression by regulatory T cells. T cell anergy, a long-lived hyporesponsive state of T cells, occurs when T cells interact with major histocompatibility complex molecules on antigen-presenting cells in the absence of co-stimulatory signals. Removal of T cells entails T cell apoptosis due to repeated stimulation of T cells without co-stimulation.
CD28, one of the classical co-stimulatory molecules in T cells, interacts with its ligands (CD80 [B7-1] and CD86 [B7-2]) expressed on professional antigen-presenting cells. Unlike solid tumors, B cell-derived lymphomas express CD80 and/or CD86, which induce T cell proliferation and prevent T cell anergy.
CLL B cells lack CD80 and CD86, but upon stimulation, levels of CD80 and CD86 increase, resulting in the appearance of antigens and activation of T cells. Moreover, lymph node-derived CLL cells exhibit higher expression of CD80 and CD86 than circulating CLL cells. These results suggest that B cell-derived tumor cells have functional co-stimulatory molecules. Thus, self-reactive T cells can be activated after breaking out of peripheral tolerance through mechanisms such as anergy and deletion.
Regulatory T cells play a critical role in regulating T cell activation during peripheral tolerance. Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), a structural homologue of CD28, is expressed on regulatory T cells and has a significantly higher affinity for CD80 and CD86 than CD28. CTLA-4 competitively inhibits CD28-CD80/CD86 signaling and suppresses the expression of CD80 and CD86, so that regulatory T cells induce self-reactive anergy and T cell inactivation.
Ipilimumab, a CTLA-4 blocking antibody, worsens pre-existing autoimmune diseases. Regulatory T cells are heterogeneous and can be unstable depending on their environment. The population of thyrogenic regulatory T cells, as a rule, retains its suppressive activity, while the population of regulatory T cells of peripheral origin can change their functional properties under inflammatory conditions. Although the role of regulatory T cells in PSP has not been studied, recent studies in FOXP3 −/− scurfy mice have shown that the absence of regulatory T cells leads to autoimmune bullous skin diseases mediated by anti-BP230 antibodies. Similar to the results of the mouse study, bullous pemphigoid, characterized by anti-BP180 and anti-BP230 autoantibodies, reportedly developed in a child with immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX) caused by a FOXP3 mutation. Thus, an imbalance of regulatory T cells may lead to the induction of paraneoplastic autoimmunity.
The pro-inflammatory cytokine interleukin-6 (IL6) is a major extrinsic factor inhibiting the differentiation of regulatory T cells. IL6 −/− mice or mice treated with an anti-IL-6R blocking antibody exhibit increased frequency of regulatory T cells and are resistant to various autoimmune diseases. In addition to the differentiation of regulatory T cells, IL-6 inhibits FoxP3 expression and the suppressive function of regulatory T cells. In addition, IL-6 promotes the differentiation and function of T follicular helper cells, which interact with B cells and aid in B cell proliferation, differentiation, and isotype switching.
Most cases of PSP showed markedly elevated serum levels of IL-6, and recent studies have shown that IL-6 is a major cause of disease progression in idiopathic multicentric Castleman disease, which has a significantly higher incidence of PSP than other neoplasms. Taken together, these results imply that IL-6 may be a critical inducer of paraneoplastic autoimmunity, although more research is needed to substantiate the link between IL-6 and autoimmunity in PSP.
Molecular mimicry
PSP can also be caused by an antitumor immune response. Tumor-specific neoantigens arise from tumor mutations. T cells in response to neoantigens can cross-react with autoantigens derived from normal epithelial proteins and thus induce autoimmunity due to molecular mimicry. Neoantigens that mimic autoantigens derived from desmosomal and hemidesmosomal proteins have not been studied in neoplasms to date, although studies have shown that several proteins, including desmoglein 3, BP180, BP230, and α6β4 integrin, are overexpressed in carcinomas of epithelial origin. Once an autoimmune response to a self-antigen begins, tissue damage can spread the activation of adaptive immune cells specific for other autoantigens, called epitope spreading. The concept of epitope spreading may explain why people with PSP exhibit autoantibodies targeting multiple autoantigens.
Directions for future research
Because it is a rare disease, PSP is still poorly understood. Although our understanding of PSP is gradually improving, the pathogenesis and etiology of this disease remain unknown. Moreover, there is a lack of effective treatment options for PSP. Additional studies in humans and animals will be required to explore the role of anti-plakin autoantibodies in disease manifestation and the mechanism of bronchiolitis obliterans.
The causes of PSP can be heterogeneous, depending on the associated malignancies; therefore, different baseline approaches are needed to understand the breakdown of immune tolerance in PSP. Currently, there is no consensus regarding the diagnostic criteria for this disease. Thus, large-scale clinical studies are needed to optimize the diagnostic algorithm and develop additional effective treatment strategies aimed at suppressing the autoimmune response.
Mechanism of bubble formation
Human skin can be figuratively described as a water-spring “mattress” covered with a kind of “wall”. The “mattress” does not participate in the formation of bubbles - only the top layer, the epidermis, suffers.
The epidermal layer consists of 10-20 cell layers, which look like bricks under a microscope. The “bricks” of the second layer of the epidermis are connected to each other by peculiar “bridges”. On top of the “wall” there are layers of cells that are no longer quite similar to cells, reminiscent of applied cream. These are scales, corneocytes, necessary for protection from mechanical, chemical and physical damage.
If, under the influence of internal or external causes, antibodies are formed that destroy the “bridges” - desmosomes between the cells of the basal layer (this is called acantholysis and can be seen under a microscope), this is true pemphigus. If tissue fluid penetrates between the basal and upper layers of the epidermis without destroying the “bridges,” it is pemphigoid. Viral pemphigus also occurs without destruction of desmosomes.
Leaf type pemphigus
The leaf-shaped type of pemphigus, in its symptoms, is a rash of the erythematous-squamous type; the blisters have thin walls and often appear on areas that were already affected. After the bubbles open, the eroded surface becomes bright red. When the surface dries out, lamellar crusts appear. Since with this form of pemphigus, blisters also form on the crusts, the affected area of the skin can become covered with a massive layer of crust due to the abundant exudate.
The leafy form of pemphigus involves the entire skin, but rarely also the mucous membranes are involved. Pemphigus leaf quickly covers the entire skin, on which blisters, erosions and fresh crusts immediately appear. Uniting with each other, the damaged areas form a large wound surface. Nikolsky syndrome will be positive even on a healthy area of skin. If pathogenic microflora attaches, sepsis will begin to develop, which is why a person’s death occurs.
Classification
Types of non-acantholytic pemphigus:
- Non-acantholytic pemphigus is benign. Pathological elements are formed exclusively in the human oral cavity. Upon examination, inflammation of the mucous membrane, as well as its slight ulceration, can be detected.
- Bullous form of non-acantholytic pemphigus. This is a benign disease that develops in both adults and children. Blisters form on the skin, but there are no signs of acantholysis. These pathological elements can spontaneously disappear without scarring.
- Cicatricial non-acantholytic pemphigus. This pemphigoid is called pemphigus of the eye in the medical literature. Most often it is diagnosed in women who have crossed the 45-year age limit. A characteristic symptom is damage to the visual apparatus, skin and oral mucosa.
Classification of true pemphigus:
- Erythematous form. This pathological process combines several diseases. Its symptoms are similar to seborrheic dermatitis, an erythematous variant of systemic lupus, as well as true pemphigus. Erythematous pemphigus in adults and children is very difficult to treat. It is worth noting that the disease is diagnosed not only in people, but also in some animals. A characteristic symptom is the appearance of red spots on the skin of the body and face, covered with crusts on top. Simultaneously with this symptom, seborrheic manifestations appear on the scalp.
- Pemphigus vulgare. This type of pathology is diagnosed in patients more often. Blisters form on the skin, but there are no signs of inflammation. If pemphigus is not treated on time, pathological elements can spread throughout the entire skin. It is worth noting that they can merge and form large lesions.
- Pemphigus foliaceus. This form received its name due to the characteristics of the pathological elements. Blisters form on the human skin, which practically do not rise above the epidermis (not tense). Crusts form on top of them, which tend to layer on top of each other. The effect of sheet material folded in stacks is created.
- Brazilian pemphigus. There are no restrictions regarding gender and age. Cases of its development have been recorded in both young children and elderly people aged 70 to 80 years. It is also possible that it may progress in middle-aged people. It is worth noting that this variety is endemic and is therefore found only in Brazil.
Symptoms
Considering that experts have identified several different types of this pathology, the symptoms of each of them will be very specific. Of course, there are a number of general trends and signs inherent in all types of the disease. This may include, for example, the wave-like course of the pathological process.
Periods of exacerbation alternate with the transition of pemphigus to a calmer stage, when the main symptoms subside or completely disappear. An important factor for the patient will be the fact that in the absence of timely diagnosis and prescription of an effective course of treatment, there is a high risk of developing severe conditions aggravated by concomitant diseases.
- The presence of crusts, ranging from pale pink soft to red dense, reminiscent of lichen;
- There is a deterioration in the general condition;
- Decreased immune response of the body;
- Formation of bubbles of varying densities;
- Also, in severe cases, separation of the layers of the epidermis is noted, and it can occur both in the lesion and away from it.
- Damage and ulcers of the mucous membrane of the mouth, nasopharynx or genitals;
- Pain when performing the act of swallowing or when eating;
- Bad breath, indicating damage to the mucous membranes;
- Hypersalivation or, in other words, increased salivation;
- In the seborrheic form, characteristic yellowish or brown-brown crusts appear on the scalp.
- Bubbles vary in appearance, ranging from flat to thin-walled, which burst with a slight touch. In their place, erosions and, subsequently, crusts form.
- In severe cases, an eroded surface of the skin may form in place of the blisters. Their feature is a tendency towards peripheral growth. Over time, such erosions occupy a large surface of the skin, causing pain and inconvenience to the patient.
- In children, manifestations of pemphigus are localized over the entire surface of the skin, including the limbs.
Experts say that with this disease, both a pure form of the pathological process and mixed forms that smoothly transform into one another can be observed. Therefore, the symptoms and signs of pemphigus in a given person may vary and indicate the presence of several types of disease.
Clinical case of pemphigus vulgaris in old age
True pemphigus is one of the most serious diseases. It accounts for 0.7 to 1% of all skin diseases [1, 4]. According to the regional dermatovenerological dispensary in Astrakhan for 2014, only 9 patients with pemphigus were treated as inpatients. Pemphigus can occur at any age. Most often, women over 40 years of age are affected; in recent years, cases of the disease among young people aged 18 to 25 have become more frequent. The most severe course is observed between the ages of 30 and 45 years [1, 4]. Pemphigus (acantholytic, or true, pemphigus) is an autoimmune disease characterized by the appearance of intraepidermal blisters on apparently unchanged skin and/or mucous membranes. The characteristic morphological basis is suprabasal blisters with acantholysis [4, 7, 8]. The etiology of pemphigus still remains unknown [1]. Currently, the leading role of autoimmune processes that develop in response to changes in the antigenic structure of epidermal cells under the influence of various damaging agents is recognized. Cell damage is possible as a result of chemical, physical, and biological factors [4]. It was found that in pemphigus, autoantibodies are directed against the surface structures of epidermal cells - keratinocytes. The binding of autoantibodies (pemphigus IgG) to glycoproteins of cell membranes (pemphigus antigens) of keratinocytes leads to acantholysis - impaired adhesion between cells and the formation of blisters. It has been shown that the complement system and inflammatory cells are not involved in this process, although the presence of complement enhances the pathogenicity of autoantibodies, and infection in areas of skin damage leads to the addition of an inflammatory process, which aggravates the patient’s condition [6]. Risk factors for the development of true pemphigus may include various exogenous and endogenous factors (including genetic predisposition). HLA-DR and HLA-DQ polymorphisms have been shown to be the basis of genetic predisposition to pemphigus (and other autoimmune diseases) [4, 6].
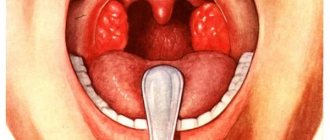
There are four clinical forms of true pemphigus: vulgar (ordinary), vegetative, foliate and erythematous (seborrheic). All clinical varieties are characterized by a long-term chronic wave-like course, leading in the absence of treatment to a disturbance in the general condition of the patients. Pemphigus vulgaris is the most common (up to 80% of all cases) [4]. In more than 50% of cases, the disease begins with damage to the mucous membranes of the oral cavity and pharynx. Small flabby blisters with serous contents that appear on unchanged mucous membranes, initially single or few in number, can be located in any area. Over time, their number increases. The blisters quickly (within 1–2 days) open, forming weeping, painful erosions with a bright red bottom or erosions covered with a whitish coating, bordered along the periphery by fragments of whitish epithelium. With further intensification of the erosion process, they become numerous, increase in size and, merging with each other, form foci of scalloped outlines. Patients experience pain when eating, talking, or swallowing saliva. A characteristic symptom is hypersalivation and a specific putrid odor from the mouth. If the larynx and pharynx are affected, the voice may be hoarse. For a long time, patients are observed by dentists or ENT doctors for stomatitis, gingivitis, rhinitis, laryngitis, etc. Damage to the mucous membranes can remain isolated from several days to 3–6 months. and more, and then the skin of the torso, limbs, and scalp is involved in the process.
Skin damage begins with the appearance of single blisters, then their number increases. The blisters are located on an unchanged, less often on an erythematous background. They are small in size, have a tense tire and serous contents. After a few days, some blisters on the skin dry out into yellowish crusts, or when the tire ruptures, bright red erosions may be exposed, releasing a thick exudate. Erosions at this stage of the disease are not painful and quickly epithelialize. The general condition of the patients remains satisfactory. The rashes that have regressed are replaced by new ones. This initial phase can last from 2-3 weeks. up to several months or even years. Then comes the generalization of the process, characterized by the rapid spread of rashes over the skin and the transition to the mucous membranes of the oral cavity and genitals, if they were not previously affected. As a result of eccentric growth due to exfoliation of the upper layers of the epidermis, the blisters increase in size, the tire becomes flabby, and the contents become cloudy or purulent. Under the weight of exudate, large blisters can take on a pear-shaped form (“Sheklakov’s pear syndrome”). The blisters spontaneously burst with the formation of extensive eroded areas of the skin. Erosions in pemphigus vulgaris are usually bright pink in color with a shiny, moist surface. The peculiarity of erosions is a tendency to peripheral growth, while generalization of the skin process with the formation of extensive lesions, deterioration of the general condition, the addition of a secondary infection, the development of intoxication and, in the absence of treatment, death are possible [1–5]. An important diagnostic sign of pemphigus vulgaris is Nikolsky’s symptom: detachment of the apparently unchanged epidermis when pressing on its surface near the bubble or even on apparently healthy skin far from the lesion [1]. There are three variants of Nikolsky's symptom, which allow us to assess the prevalence of acantholysis. In the first case, when the tire of a burst bladder is pulled, the epidermis peels off beyond its borders. In the second option, the upper layer of the epidermis peels off, and an erosive surface is formed if healthy skin is rubbed between two blisters. The appearance of erosion after rubbing healthy skin in a place near which there are no bullous elements indicates the presence of the third variant of Nikolsky’s symptom [5]. A modification of Nikolsky's symptom is the Asbo-Hansen phenomenon: finger pressure on the tire of an unopened bladder increases its area due to further stratification of the acantholytically altered epidermis with vesical fluid. In the initial phase of pemphigus vulgaris, Nikolsky’s symptom is not always detected, and even then only in the form of a marginal one. When the process is generalized, it is positive in all patients in all modifications [3]. Diagnosis of true pemphigus is based on the totality of the results of clinical, cytological, histological and immunological examination. The clinical picture of the disease, the presence of a positive Nikolsky symptom and its modification, the “pear” phenomenon described by N.D. are taken into account. Sheklakov, which are based on the phenomenon of acantholysis. Cytological examination reveals acantholytic cells (Tzanck cells) in impression smears from erosions and blisters after staining using the Romanowsky-Giemsa method (Tzanck test). The presence of Tzanck cells in the blisters is not pathognomonic, but a very important diagnostic sign of the disease.
Histological examination reveals the intraepidermal location of cracks and blisters [1, 4]. A necessary condition for a qualified diagnosis of true pemphigus is an immunofluorescence study. By means of indirect immunofluorescence, antibodies against components of the epidermis are detected when treated with luminescent anti-IgG human serum. Using direct immunofluorescence, IgG antibodies are detected in skin sections, localized in the intercellular spaces of the spinous layer of the epidermis [1]. Laboratory data (anemia, leukocytosis, increased ESR, proteinuria, hypoalbuminemia, decreased sodium excretion in urine, etc.) play a certain supporting role, allowing one to assess the severity of the process [3]. Differential diagnosis is carried out with Lever's bullous pemphigoid, Dühring's dermatitis herpetiformis, chronic benign familial pemphigus Gougereau-Hailey-Hailey, lupus erythematosus, seborrheic dermatitis, Lyell's syndrome, chronic pyoderma vegetans [4].
Treatment of true pemphigus has so far caused great difficulties. Since the main links of pathogenesis are interpreted from the standpoint of autoimmune pathology, all existing therapeutic measures are reduced to immunosuppressive effects on autoallergic processes through the use of corticosteroid and cytostatic drugs [1]. The introduction of corticosteroids into the treatment of pemphigus reduced mortality among patients from 90 to 10% [6]. Pemphigus is one of the few diseases in which corticosteroids are prescribed for health reasons, and existing contraindications in these cases become relative. The positive effect of glucocorticoids is explained primarily by the blockade of key stages of the biosynthesis of nucleic acids and proteins, shutdown of the afferent phase of immunogenesis, reduction of lymphoid organs, destruction of medium and small thymic lymphocytes, and inhibition of the formation of immune complexes. It is also believed that corticosteroids have a stabilizing effect on lysosome membranes and inhibit the synthesis of autoantibodies [1]. Typically, pemphigus vulgaris and pemphigus foliaceus are the most severe, therefore, for these clinical forms, the highest doses of glucocorticosteroids are prescribed (from 60–100 to 150–300 mg/day of prednisolone equivalent) [1,4]. The dose of prednisolone is selected taking into account the prevalence of the rash and the severity of the disease. It should be at least 1 mg/kg/day. The daily dose is distributed in such a way that 2/3 falls in the early morning hours (preferably after meals), and 1/3 in the afternoon (12–13 hours). If the patient’s condition is particularly severe, higher doses of prednisolone are prescribed – up to 300 mg/day [4]. At high doses, prednisolone can be partially replaced by parenteral administration or betamethasone (it is possible to use prolonged forms once every 7-10 days). Long-term use of corticosteroid drugs leads to the development of serious complications and side effects, and if they are quickly discontinued, the so-called withdrawal syndrome occurs, and the disease recurs. Therefore, it is necessary to correct and prevent side effects caused by long-term use of glucocorticosteroids. In order to prevent withdrawal syndrome, it is recommended to stop taking medications or reduce their daily dose carefully and gradually. Initially, a reduction in the dose of glucocorticosteroids is possible by 1/4–1/3 of the maximum initial dose after achieving a clear therapeutic effect (cessation of the appearance of new blisters, active epithelization of erosions), which usually occurs after 2–3, sometimes after 4 weeks. Then the dose of prednisolone is gradually, slowly, over several months, reduced to maintenance. The daily dose of the hormone is gradually reduced, approximately once every 4–5 days by 2.5–5 mg of prednisolone until the minimum maintenance effective dose of the corticosteroid is reached, the administration of which ensures remission of the disease.
In the future, a maintenance dose of corticosteroids is recommended to be administered alternately. However, periodically (every 4–6 months) it should be reduced by 2.5 mg prednisolone equivalent. Thus, by reducing the maintenance dose, it is possible to reduce the amount of hormone administered by 3-4 times compared to the initial maintenance dose. The maximum permissible minimum maintenance dose can vary from 2.5 to 30 mg/day. Typically, patients with pemphigus receive glucocorticosteroids for life, and sometimes their use can be abandoned [1, 4]. The addition of second-line drugs to therapy is indicated to increase the effect of treatment, reduce the side effects of corticosteroids, and also to prevent relapses during their gradual withdrawal. Adjuvant therapy includes azathioprine, methotrexate, cyclophosphamide, mycophenolate mofetil, intravenous immunoglobulin and dapsone [8]. The combined use of cytostatic and immunosuppressive drugs with corticosteroids allows one to achieve good therapeutic results in a shorter time and with lower daily doses of corticosteroids. Many drugs, such as alkylating agents and antimetabolites, have cytostatic properties. Of the alkylating agents, cyclophosphamide is the most widely used in the treatment of pemphigus. This drug is capable of entering into alkylation reactions with certain groups of proteins and nucleic acids of the cell, inhibiting various enzyme systems and dramatically disrupting the vital activity of cells, primarily highly active and lymphoid ones. Antimetabolites, which include purine base antagonists (azathioprine) and folic acid antagonists (methotrexate), resemble the structure of natural cell metabolites and, by competing with them, disrupt intracellular metabolism. The consequence of this is the accumulation of substances toxic to cells, leading to the death of cells, primarily actively proliferating ones. Azathioprine is prescribed at a dose of 1.5–2 mg/kg/day in 2–4 divided doses in combination with steroids. Methotrexate is administered intramuscularly at 10–20 mg (with good tolerance up to 25–30 mg) 1 time per week (for a course of 3–5–8 injections). Cyclophosphamide is administered orally at 100–200 mg/day, the duration of therapy is determined individually. During treatment, monitoring of blood tests (general and biochemical) and urine is necessary.
If the therapeutic effectiveness of glucocorticosteroids is insufficient and there are contraindications to the use of cytostatics, immunosuppressants are prescribed. Cyclosporine A for the treatment of patients with true pemphigus is used in combination with corticosteroid drugs, and the daily dose of corticosteroids is reduced by 3-4 times and corresponds to 25-50 mg of prednisolone equivalent. The daily dose of cyclosporine A in the complex therapy of patients with true pemphigus in the acute stage should not exceed 5 mg per 1 kg of the patient’s body weight and averages 3–5 mg/kg/day. This takes into account the clinical picture, severity and prevalence of the disease, the patient’s age, and the presence of concomitant diseases. For the first 2 days, to assess the tolerability of the drug, cyclosporine A is prescribed in half the dose, subsequently the daily dose is divided into 2 doses - morning and evening with an interval of 12 hours. The daily dose of cyclosporine A begins to be reduced after intensive epithelization of existing erosions. Typically, a loading dose is taken on average for 14–20 days, followed by a gradual reduction in the daily dose of the drug to 2–2.5 mg per 1 kg of the patient’s body weight. Complete cleansing of the skin should not be considered the final goal of treatment. After achieving remission, the patient should continue to take the minimum effective maintenance dose of cyclosporine A, which should be selected individually. At this dosage, the drug can be used for a long time (2–4 months) as maintenance therapy. Currently, treatment with immunosuppressants is not generally accepted [1, 4]. Aniline dyes, corticosteroid creams with an antibacterial or antimycotic component, and aerosols are used locally [3]. For additional treatment of pemphigus, extracorporeal detoxification methods (hemosorption, plasmapheresis) are successfully used [1]. Despite the successes of domestic and foreign researchers in clarifying the mechanisms of pathogenesis and improving treatment methods for patients with true pemphigus, the problem of pemphigus remains relevant and is due to the severity of the disease, its incurability and potential mortality [1].
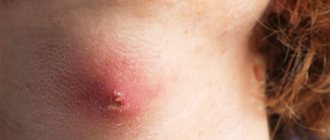
We present a clinical case demonstrating the difficulties of differential diagnostic search when diagnosing pemphigus. Patient Zh., born in 1945, has been ill since the fall of 2014, when rashes first appeared on the scalp. I treated myself, used ointments with antibiotics without effect. In May 2015, an exacerbation of the skin process began. The rash has spread to the face and torso. He was observed in the clinic at his place of residence, and due to the torpidity of treatment, he was sent to the regional oncology clinic, where a pathohistological examination of the scalp was carried out. After examination, a diagnosis was made: multiple scalp cancer. Т1N0М0. Radiation therapy is recommended. Within 2 weeks. the process on the skin has spread significantly: elements have appeared on the chest, back, and upper limbs. Serous-purulent discharge was noted on the scalp, and the erosions took on a draining character. Taking into account the change in the clinical picture, he was sent for consultation to the regional dermatovenerological clinic with a diagnosis of pemphigus vulgaris. As a result of examination in the department, this diagnosis was clinically and laboratory confirmed. At the same time, pathohistological preparations were reviewed at the oncology clinic, and the diagnoses of cancer and basal cell carcinoma of the scalp were removed as erroneous. From the anamnesis it is known that the patient has a burdened premorbid background. In 2012, he suffered a small-focal myocardial infarction. Suffering from chronic bronchitis. Upon examination, a widespread pathological process was revealed. On the skin of the scalp, face, neck, torso and upper extremities there were multiple bright red erosions with serous and serous-purulent discharge. Some of the erosions were covered with dense crusts of gray-yellow color (Fig. 1–3). On an erythematous background, there were blisters of various sizes with a flabby cap and cloudy contents. Nikolsky's symptom is positive. During a laboratory examination, smears from blisters on the forearms revealed single acantholytic cells in a specimen with coarse nuclei. Epithelial cells with signs of atypia (enlarged coarse nuclei, binucleate cells) up to 8–10–12 per field of view were identified. During bacteriological examination of discharge from erosions, staphylococcus was isolated. According to the pathomorphological study, the morphological picture of pemphigus was revealed - a bubble with serous fluid and acantholytic cysts with purulent inflammation along the periphery was found in the epidermis. When reviewing the drugs at the oncology center, the morphological picture of pemphigus was confirmed and no tumor growth was detected. A general clinical examination showed an increase in ESR and a sharply positive C-reactive protein. Fibrogastroduodenoscopy revealed erosive antrum gastritis, erosive and ulcerative bulbitis and duodenitis. Small (up to 0.2 cm) acute ulcers of the bulb and the upper horizontal part of the bulb were found.
Taking into account the generalized process of skin lesions, parenteral administration of prednisolone at a dose of 90 mg was prescribed with a gradual transition to oral administration of the drug at a dose of 30 mg. He also received detoxification, antibacterial and antifungal systemic therapy and local treatment using antiseptics, reparatives and anti-inflammatory drugs. Due to the presence of erosive gastritis, erosive-ulcerative bulbitis, and also because of taking prednisolone, the patient was given antisecretory therapy. During therapy for 3 weeks. positive dynamics were noted. On the skin of the scalp, neck, chest, and back, erosions were completely epithelialized, and the crusts fell off (Fig. 4–6). He was discharged for outpatient treatment with recommendations to reduce the dose of prednisolone by 1/4 tablet every 7–10 days under the supervision of a dermatovenerologist at the place of residence.
The development of pemphigus in a 70-year-old man with a latent onset of the disease may have been the reason for late diagnosis and, consequently, untimely initiation of therapy. After correct interpretation of clinical and morphological data and complex therapy, a positive result was achieved.
Literature 1. Matushevskaya E.V. Pemphigus // Russian Medical Journal. 1997. No. 11. 2. Mordovtsev V.N., Mordovtseva V.V., Alchangyan L.V. Erosive and ulcerative skin lesions // Consilium Medicum. 2000. No. 5. 3. Paltsev M.A., Potekaev N.N., Kazantseva I.A., Kryazheva S.S. Clinical and morphological diagnosis and principles of treatment of skin diseases. Guide for doctors. M.: Medicine, 2010. 4. Kubanova A.A., Kisina V.I., Blatun L.A., Vavilov A.M. and others. Rational pharmacotherapy of skin diseases and sexually transmitted infections: A guide for practitioners / edited by. ed. A.A. Kubanova, V.I. Kisina. M.: Litterra, 2005. 882 p. (Rational pharmacotherapy: ser. handbook for practicing physicians; vol. 8). 5. Rubins A. Dermatovenerology. Illustrated guide. M.: Panfilov Publishing House, 2011. 368 p. 6. Svirshchevskaya E.V., Matushevskaya E.V. Immunopathogenesis and treatment of pemphigus // Russian Medical Journal. 1998. No. 6. 7. Kumar R., Jindal A., Kaur A., Gupta S. Therapeutic Plasma Exchange-A New Dawn in the Treatment of Pemphigus Vulgaris // Ind. J. Dermatol. 2015. Vol. 60(4). P. 419. doi: 10.4103/0019-5154.160509. 8. Quaresma MV, Bernardes-Filho F., Hezel J. et al. Dapsone in the treatment of pemphigus vulgaris: adverse effects and its importance as a corticosteroid sparing agent // An. Bras. Dermatol. 2015. Vol. 90 (3 Suppl. 1). R. 51–54.
Diagnostics
Experts say that a correct diagnosis can be made based on a comprehensive examination of the patient, which includes several important stages:
- Examination of the patient for the presence of a clinical picture. At this point, the doctor establishes the nature of the lesions, their localization, the degree of development of the disease, etc.
- Cytological analysis necessary to establish the presence of acantholic cells in smears of biomaterial.
- Carrying out the Nikolsky test, which allows to differentiate pemphigus from similar pathological processes.
- Method of direct immunofluorescence. This study allows us to detect the presence of immunoglobulin in the intercellular substance of the epidermis.
- A histological study, which is based on a technique for detecting crevices and other damage within the epidermis.
Only the totality of all the results makes it possible to make an accurate diagnosis and prescribe an effective course of treatment, leading to the patient’s recovery.
Vegetating type of pemphigus
The vegetative type of pemphigus is benign. Patients can feel well for many years. Bubbles are located around the holes, as well as in the area of skin folds. When the bubbles open, they reveal erosions, at the bottom of which soft vegetations appear that have a fetid odor. Such vegetations are covered with serous-purulent or simply serous fluid. Pustules can be seen along the contour of the neoplasms, so pemphigus vegetans must be distinguished from the chronic form of pyoderma. Nikolsky syndrome will be positive only near the affected areas of the skin, however, in the terminal stages, pemphigus vegetans is similar to pemphigus vulgaris in its clinical manifestations.
Treatment of viral pemphigus
Treatment of viral pemphigus involves the use of the following systemic drugs:
- cytostatics stop the division of immune cells: Sandimmune, Azathioprine, Methotrexate;
- antiviral: Viferon, Laferon, Cycloferon;
- glucocorticosteroids: Dexamethasone, Prednisolone;
- antipyretics: Ibuprofen, Paracetamol, Nimesil, Mefenamic acid;
- antihistamines relieve itching: Cetrin, Diazolin, Fenistil.
For external treatment of affected skin areas, the following may be prescribed:
- antimicrobial local anesthetics for irrigating the oral cavity if viral pemphigus has affected the child’s mucous membranes: Forteza, Orasept;
- antiseptics: Chlorhexidine, Methylene blue, Miramistin;
- combination preparations of antiseptics and anesthetics: Oflokain, pharmaceutical talkers;
- antipruritic lotions made from nettle juice, aloe, and walnut oil.
Since children with this diagnosis are usually treated in a hospital setting, to enhance the therapeutic course, therapeutic procedures can be carried out aimed at clearing the blood of antibodies:
- plasmapheresis - replacement of the liquid part of the blood with similar solutions without microbes, immune complexes and antibodies;
- hemosorption using a carbon filter.
Only a doctor can tell how to treat viral pemphigus, because in each individual case it can acquire some special features. As for other forms of pemphigus, the therapeutic course for them is also determined individually.
Treatment
How to treat pemphigus in a child? The main drugs today are hormones from the group of glucosteroids. In this case, systemic therapy is carried out without age restrictions.
Treatment of pemphigus has several main directions:
- preventing the appearance of new blisters and erosions;
- healing of affected areas of the skin.
Glucosteroids are administered in increased dosages. Due to this technique, the intensity of the formation of new foci decreases and the restoration process begins in existing erosions. This process takes about 2 weeks. Subsequently, the patient is transferred to maintenance hormonal therapy. The drug does not change, but the dosage is significantly reduced.
Viral pemphigus in children treatment is aimed at suppressing the virus. Pemphigus vulgaris requires the administration of a larger volume of drugs than foliaceus. The transition to maintenance therapy is carried out gradually. Moreover, the vast majority of patients are forced to take daily injections of maintenance doses of glucosteroid drugs throughout their lives.
Children should take hormonal medications simultaneously with calcium and vitamin D. The effectiveness of treatment increases due to the use of immunosuppressive drugs from the first days of treatment. These drugs have a depressing effect on the activity of the body's immune system.
Early stages of treatment often involve the use of procedures aimed at clearing the blood of aggressive antibodies. Such procedures include hemodialysis and plasmapheresis. The skin must be treated with antiseptics and special ointments to reduce the risk of infection.
Treatment of pathology is daily and lifelong. Sometimes there may be breaks between relapses of the disease.
Baby care
The diagnosis of pemphigus requires special, attentive and daily care for the child. The organization of a baby’s life directly affects his life expectancy. The initial stage of treatment takes place within the walls of the hospital. After discharge, the child should receive all necessary medications in the prescribed volume and time of administration. Parents must learn how to give injections, because the daily services of a hired nurse will negatively affect the family budget.
Every day your child needs to treat blisters and eczema on the skin. Processing is carried out using aniline dyes. This group of agents has the widest spectrum of action on various microbes, including staphylococcus. Erosion and crusts formed are treated with corticosteroid-based ointments.
The presence of infectious signs in the form of pus, inflammation, swelling means the start of using antibiotic ointments. Large affected areas require the use of sterile dressings to help avoid further injury. Dressings are changed at least 2 times a day. For small affected areas, the relevance of bandages is associated with the child’s high motor activity.
The appearance of pain requires taking painkillers and consulting a specialist. The presence of damaged areas in the oral cavity is associated with mandatory rinsing of the mouth with antiseptic agents. A useful and effective means of care is taking baths with antiseptic agents. It is mandatory to take vitamin and mineral complexes consisting of vitamin E, calcium, magnesium and folic acid.
The diet also requires enrichment with vitamins and minerals. Meals are provided on a fractional and frequent basis, at least 6 times a day. This diet plan is especially important when the esophagus and mucous membranes in the mouth are affected. Diet therapy is based on the complete exclusion of salt and increasing the amount of protein. Registration at a dispensary and systematic visits to a dermatologist are required - at least twice a year and urgently in case of relapse. The use of immunosuppressive therapy imposes restrictions on vaccinations.
How to treat other forms of pemphigus?
The treatment process for pemphigus is quite complicated. Therefore, self-medication of this type of disease is under no circumstances acceptable. The disease progresses rapidly, affecting large areas of the skin, which leads to disruption of the internal organs.
Treatment of pemphigus is mandatory in a dermatological hospital. First of all, corticosteroid drugs, cytostatics and other drugs are prescribed to alleviate the course of the disease and the life expectancy of patients.
The drugs must first be taken in large doses. At the same time, pay attention to blood and urine sugar levels, monitor blood pressure and observe personal hygiene rules. With frequent changes of bed linen and underwear, secondary infection is prevented.
Who's at risk
It is quite difficult to identify a specific group that is at risk of getting pemphigus. Thus, symptoms can manifest at any age, including infancy: the diagnosis of “pemphigus of newborns” is known. In old age, people can also observe manifestations of this pathology. However, statistically, people aged 40-45+ often suffer from it. In addition, those who have a hereditary predisposition should also be wary of developing an infection.
Rashes on the face. What are the causes of acne? More details
It is clear that such a disorder requires timely consultation with a doctor, since if therapy is started at the wrong time (or not started at all), there can be serious complications, and death is also possible.
There are contraindications, you should consult your doctor
Medicines for the treatment of pemphigus
The patient is advised to take glucocorticoids in high doses. The following drugs can be used for this:
- Metipred;
- Prednisolone;
- Dexamethasone;
- Polcortolon.
When symptoms begin to regress, the doses of these drugs are gradually reduced to the minimum effective. Patients with pathologies of the gastrointestinal tract are prescribed long-acting glucocorticoids:
- Metipred-depot;
- Diprospan;
- Depo-Medrol.
Treatment with hormonal drugs can cause a number of complications, but they are not a reason to discontinue corticosteroids. This is explained by the fact that refusal to take them can lead to relapses and progression of pemphigus.
Possible complications during treatment:
- acute psychosis;
- arterial hypertension;
- depressive states;
- insomnia;
- increased excitability of the nervous system;
- steroid diabetes;
- thrombosis;
- obesity;
- angiopathy;
- erosions or ulcers of the stomach and/or intestines.
If the patient’s condition sharply worsens while taking corticosteroids, the following measures may be recommended:
- diet: limiting fats, carbohydrates and table salt, introducing more protein and vitamins into the diet;
- drugs to protect the gastric mucosa: Almagel, etc.
In parallel with glucocorticoids, cytostatics and immunosuppressants are prescribed to increase the effectiveness of therapy and the possibility of reducing doses of hormonal agents.
The following medications can be used for this:
- Sandimmune;
- Methotrexate;
- Azathioprine.
To prevent electrolyte imbalance, the patient is recommended to take calcium and potassium supplements. And for secondary infection of erosions - antibiotics or antifungal agents.
The ultimate goal of drug therapy is to make the rash disappear.
Preventive measures
There are no specific measures to prevent the development of pathology. The higher the level of immune protection, the less chance of dermatological diseases.
Important:
- control the nature of chronic diseases;
- strengthen immunity;
- maintain personal hygiene;
- Healthy food.
Measures to prevent pemphigus in newborns:
- change your underwear more often;
- Caring for newborns with pustular skin lesions is prohibited;
- Take regular care of your child’s skin;
- strengthen the immune system of weakened children;
- daily wet cleaning and ventilation of the room are required.
If you notice any rashes on the skin, the formation of pustules and blisters, immediately contact a dermatologist.
Forecast
The prognosis for acantholytic pemphigus is conditionally unfavorable. On the one hand, in the absence of effective treatment, there is a high probability of complications and death.
On the other hand, patients with pemphigus are forced to take glucocorticosteroids for a long time, and sometimes for life, which is fraught with the development of side effects. But hasty refusal of drugs leads to immediate relapse of the disease. Glucocorticosteroids do not eliminate the cause of the disease, but inhibit the pathological process and prevent its progression.
2. Reasons
As stated above, the etiopathogenesis of acantholytic pemphigus has not yet been precisely established.
Various hypotheses have been put forward and considered in this regard (viral, metabolic, neurogenic, toxic, hormonal, etc.), but convincing evidence has been obtained only that autoimmune mechanisms play a leading role in the development of pemphigus. However, the root causes of such an attack from one's own immunity, trigger factors and risk factors (with the exception of being female or belonging to a certain genetic line) are still unknown.
Visit our Dermatology page