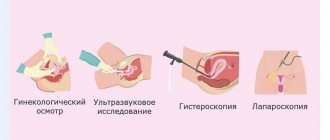
| Iodine | |
| Atomic number | 53 |
| Appearance of a simple substance | |
| Properties of the atom | |
| Atomic mass (molar mass) | 126.90447 a. e.m. (/mol) |
| Atomic radius | n/a |
| Ionization energy (first electron) | 1,008.3 (10.45) kJ/mol () |
| Electronic configuration | [Kr] 4d10 5s2 5p5 |
| Chemical properties | |
| Covalent radius | 133 |
| Ion radius | (+7e) 50 (-1e) 220 |
| Electronegativity (Pauling) | 2,66 |
| Electrode potential | 0 |
| Oxidation states | 7, 5, 3, 1, -1 |
| Thermodynamic properties of a simple substance | |
| Density | 4,93 /³ |
| Molar heat capacity | 54.44[1]/(mol) |
| Thermal conductivity | (0,45) /(·) |
| Melting temperature | 386,7 |
| Heat of Melting | 15.52 (II) kJ/mol |
| Boiling temperature | 457,5 |
| Heat of vaporization | 41.95 (II) kJ/mol |
| Molar volume | 25.7 ³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | orthorhombic |
| Lattice parameters | 7,720 |
| c/a ratio | n/a |
| Debye temperature | n/a |
| I | 53 |
| 126,90447 | |
| 5s25p5 | |
| Iodine | |
Iodine
,
iodine
(from ancient Greek ιώδης, iodes - “violet”) is an element of the main subgroup of the seventh group, the fifth period of D. I. Mendeleev’s periodic system of chemical elements, with atomic number 53. Denoted by the symbol I (Latin Iodum). A chemically active non-metal, belongs to the group of halogens. The simple substance iodine (CAS number: 7553-56-2) under normal conditions is black-gray crystals with a violet metallic luster; it easily forms violet vapors with a pungent odor. The substance molecule is diatomic (formula I2).
In medicine and biology, this substance is usually called iodine.
(for example, “iodine solution”), in the periodic table and chemical literature the name
iodine
.
History of discovery
Iodine was first obtained in 1811 by the Frenchman Courtois. He was a chemist, and at the same time the owner of a saltpeter factory. He obtained soda for making saltpeter from the ash of seaweed. When Courtois added concentrated sulfuric acid to the ash brine, a reaction began, releasing purple fumes that smelled like chlorine.
It was iodine vapor. True, rumor attributes the discovery not to Courtois himself, but to his cat. Allegedly, the animal accidentally knocked over a vessel with acid onto the ash brine.
In 1813-14, French and English chemists, Gay-Lussac and Davy, identified a new element and named it iodine from the Greek ioides, violet. Literally immediately after its discovery, in the 20s of the 19th century, the beneficial properties of iodine as a medicine in the treatment of thyroid diseases were studied.
It is noteworthy that until the middle of the last century the substance was called iodine, and in the periodic table it was designated by the letter J, Jodum. Then they decided to designate the element as iodine, and the letter J was replaced by I.
However, both in Russian and in some European languages, along with the generally accepted term, they continue to use the old one. The name “iodine” is used colloquially, as well as to refer to medications and biological processes.
The importance of iodine in our body
Compared to other microelements, also important but not always strictly necessary, iodine is essential for the proper functioning of our body . While most microelements only speed up biological processes, a lack of iodine threatens to stop the vital functions of our body.
Not only does some reaction depend on its presence, it participates in the functioning of the entire organism. Its role is especially significant in the work of one of the main regulators of metabolic processes in our body - the thyroid gland .
Physical and chemical properties
In Mendeleev’s periodic table of elements, I is located in group 17 of period 5 at number 53. Its atomic mass is 127. It is noteworthy that it was once 129 due to a heavier radioactive isotope. However, over billions of years, this unstable isotope has almost completely decayed, and there is almost none left.
Group 17 is also called halogens. In addition to iodine, the group of halogens includes fluorine, bromine, chlorine, and astatine. A distinctive feature of halogens: they have 7 electrons rotating in their outer orbit, and 1 electron is missing until complete completion. They attach this electron from atoms of other elements when interacting with them.
Therefore, halogens are usually monovalent and are strong oxidizing agents. But, the larger the atom size, the weaker the oxidizing ability. Therefore, for iodine it is lower than for many other halogens - fluorine, chlorine and bromine. However, iodine is a fairly active chemical element.
It reacts with alkalis, some acids, as well as with metals and non-metals, incl. and with their “relatives” halogens. In most cases, the valence of iodine is 1. However, under certain conditions it can be 3, 5, 7.
With hydrogen and oxygen, I participates in the formation of acids: iodic, iodic, hydroiodic, iodic, iodic. It dissolves poorly in water, and well in alcohol, ether, benzene, and other organic solvents. A characteristic reaction of iodine with starch is that it turns blue. In this regard, iodine can be considered as an indicator of starch.
The iodine molecule is formed by two atoms, I2. Externally, these are dark gray crystals with a violet sheen. The density of iodine is 4.94 g/cm3, and the melting point is 113.50C. True, under normal pressure and slow heating, solid iodine immediately passes into the gaseous state, bypassing the liquid state. This produces violet vapors with a specific odor.
However, in nature, iodine does not occur in free form due to its high chemical activity, but only in the form of compounds. In general, the iodine content in the earth's crust is low, only 4 x 10-5%. According to this indicator, it ranks 60th among all elements of the periodic table. However, iodine compounds, iodides, despite their small quantities, are ubiquitous.
They are dissolved in sea water, as well as in deep waters that flow to the surface from thermal springs and oil wells. One ton of sea water contains about 20-30 mg of I. Many marine organisms, algae, have the unique ability to extract this element from water, convert it into an digestible form, and accumulate it in their own tissues.
Physiological action
In the human body, iodine is contained in microdoses, no more than 20-25 mg. Of this total, a significant portion of iodine, about 15 mg, is found in the thyroid gland. The condition and function of our thyroid gland is inextricably linked with iodine. The thyroid gland, being an organ of the endocrine system, affects almost all physiological processes.
It exerts this influence through the thyroid hormones it produces: thyrocalcitonin, triiodothyronine, and thyroxine. Thyroid calcitonin, or simply calcitonin, ensures the exchange of calcium and phosphorus and promotes their deposition in bones. But iodine has no merit here - this microelement is not part of calcitonin.
But two other hormones, triiodothyronine and thyroxine, contain iodine in their structure. The triiodothyronine molecule contains 3 molecules of I. Therefore, it is usually designated T3. Thyroxine, also called tetraiodothyronium, contains 4 iodine molecules and is designated T4. Thus, I serves as a building block for thyroxine and triiodothyronine, and without it the synthesis of these hormones is impossible.
Thyroid hormones, and, accordingly, iodine, have a diverse effect on metabolic processes, on the condition and function of tissues and organs. In general terms, this effect is characterized by the acceleration of metabolic processes and catabolism, the breakdown of proteins, fats, and glycogen. As a result, energy is released, which is spent on the needs of the body. Energy requirements increase during stressful situations:
- physical and mental stress
- hypothermia
- negative emotions
- infectious and non-infectious (somatic) diseases
- past illnesses and injuries
- avoiding situations involving imminent danger.
And here iodine in thyroid hormones comes to the rescue. Protein catabolism (proteolysis) in skeletal muscles increases the strength of muscle contractions and provides endurance during physical work.
Under the influence of thyroid hormones, not only the breakdown of proteins occurs, but also their formation. Protein synthesis is provided by RNA. In turn, thyroid hormones, along with other factors, participate in the formation of RNA. The synthesis of proteins in combination with the acceleration of metabolic processes leads to the fact that tissues damaged as a result of injuries and inflammatory processes are restored more quickly.
In addition to protein, muscles contain glycogen. It is a kind of carbohydrate polymer consisting of monomer units, glucose. The breakdown of glycogen (glycogenolysis) in muscles is also accompanied by the release of energy, which is spent on physical work. However, most of the glycogen is stored in the liver. This organ is a depot, a kind of warehouse, for glycogen. From here, glycogen is used as needed.
The breakdown of liver glycogen under the influence of thyroid hormones is accompanied by the release of glucose into the blood. Thus, iodine maintains glycemia (blood glucose level) at the proper level. Subsequently, glucose from the blood plasma is transported into the cell, where it is burned in a cycle of biochemical reactions (Krebs cycle) to produce energy in the form of ATP molecules.
Not only physical, but also mental activity is increased by thyroid hormones. After all, a certain amount of glycogen is contained in the CNS (central nervous system) - in the brain. In addition, thyroid hormones improve the conduction of impulses along nerve fibers. Thanks to this, thinking improves and attention is concentrated. Thyroid hormones, along with other factors, ensure the perception and memorization of incoming information.
Even more energy is produced by the breakdown of fats (lipolysis). In this regard, iodine-containing thyroid hormones should be considered super fat burners. They prevent the appearance of fatty deposits under the skin, prevent obesity, and normalize body weight. Therefore, I is sometimes called the slimming microelement. The breakdown of fats is accompanied by the release of heat. Heat production plays a critical role in maintaining body temperature within physiological limits and in preventing hypothermia.
By breaking down fats, thyroid hormones reduce the amount of low-density cholesterol. It is one of the lipids (fat-like substances). Low-density cholesterol is used to build atherosclerotic plaques. Thus, thyroid hormones prevent the development of atherosclerosis and related conditions, primarily myocardial infarction and cerebral stroke.
In addition, thyroid hormones regulate blood pressure (BP). But this regulation is ambiguous. As in skeletal muscles, metabolic processes are accelerated in the cardiac muscle (myocardium). As a result, the strength and frequency of heart contractions increases. On the one hand, tissue blood supply and oxygen delivery are improved, congestive heart failure and oxygen deficiency (hypoxia) are prevented.
On the other hand, systolic blood pressure, called “upper” blood pressure, increases. However, thyroid hormones expand the vascular lumen and further improve blood circulation. At the same time, diastolic or “lower” blood pressure decreases.
Under the influence of thyroid hormones, the formation of red blood cells in the red bone marrow is accelerated. This further improves tissue oxygen transport. The formation of not only red blood cells, but also white blood cells is stimulated by thyroid hormones.
Under their influence, the phagocytic activity of leukocytes increases and the production of antibodies increases. The body becomes resistant to the action of pathogenic (disease-causing) bacteria, fungi, and viruses. In addition, iodine itself has a detrimental effect on pathogenic microorganisms.
Iodine stimulates not only the cardiovascular and immune systems, but also the digestive system. Under the influence of thyroid hormones, peristalsis of the smooth muscles of the gastrointestinal tract (gastrointestinal tract) increases. These same hormones stimulate the secretion of digestive juices.
As a result, food moves faster and is better digested. The trace element improves the digestive and detoxification function of the liver. It has been established that under the influence of thyroid hormones salts of heavy metals and some other toxins are neutralized.
Along with other organs, iodine improves blood circulation in the kidneys and stimulates urine output. Thyroid hormones regulate sodium balance, prevent fluid retention in the body, and prevent the appearance of edema. It is noteworthy that iodine acts not only as part of thyroid hormones, but also as part of some other proteins in organs and tissues.
Thus, iodine increases the strength and elasticity of the skin, slows down the skin aging process. The trace element stimulates hair growth, prevents hair loss, and improves visual acuity. The effect of iodine is not limited to the thyroid gland alone.
After all, our thyroid gland is closely interconnected with other endocrine units. It is directly subordinate to the brain structures, pituitary gland and hypothalamus. But the gland itself regulates the function of the hypothalamic-pituitary system according to the feedback principle.
Indirectly, the thyroid gland affects the function of the adrenal glands. Thyroid hormones increase adrenaline levels. This hormone additionally stimulates the central nervous system and cardiovascular system. Thyroid hormones directly or indirectly improve the absorption of many vitamins, in particular vit. E, D, A, some B vitamins.
The most pronounced influence of thyroid hormones is on the function of the male and female reproductive systems. Under their influence, cholesterol is synthesized in the liver. This is not the “bad” low-density cholesterol that leads to atherosclerosis. This is “good” high-density cholesterol, which strengthens cell membranes and vessel walls and ensures the absorption of nutrients. The same cholesterol serves as the raw material for steroid hormones.
The hormones of the adrenal cortex, male (testosterone) and female (estrogens, progesterone) sex hormones have a steroid structure. Thus, the thyroid gland, through triiodothyronine and thyroxine, ensures puberty and the formation of primary and secondary sexual characteristics.
The formation of full-fledged sperm capable of fertilization in men, maturation of follicles and ovulation during a normal menstrual cycle in women - all this is largely due to iodine. Without iodine, conception is impossible. In the same way, a normal pregnancy is impossible.
The trace element is involved in embryogenesis - the formation of organs and systems in the embryonic period. Its role is especially great in the formation of the brain. After birth, I, together with other vitamins and minerals, ensures complete lactation. In the future, iodine in the composition of thyroid hormones is responsible for proper physical and mental development.
Toxicity
Iodine is a toxic substance. Lethal dose 2-3 g. Causes damage to the kidneys and cardiovascular system. When inhaling iodine vapor, a headache, cough, runny nose appears, and possibly pulmonary edema. Contact with the mucous membrane of the eyes causes lacrimation, eye pain and redness. If ingested, general weakness, headache, vomiting, diarrhea, brown coating on the tongue, heart pain and increased heart rate appear. After a day, the kidneys become inflamed and blood appears in the urine. If left untreated, the kidneys may fail within 2-3 days and myocarditis may occur. Without treatment, death occurs.
- Thyroiditis
Daily requirement
The iodine requirement is from 2 to 4 mcg per 1 kg of body weight. By age category, this indicator looks like this:
| Category | Daily value, mcg |
| Children under 2 years old | 50 |
| Children 3-5 years old | 60-90 |
| Children 6-8 years old | 90-120 |
| Children 9-13 | 120 |
| Teenage boys | 150 |
| Teenage girls | 150 |
| Adult men | 180 |
| Adult women | 150 |
| Pregnant and nursing | Up to 300 |
The need for iodine increases in: stressful situations (physical activity, illness), taking medications that suppress thyroid function. Among these drugs:
- some nonsteroidal anti-inflammatory drugs
- glucocorticoids
- interferons
- beta blockers
- heparin
- lithium salts
- specific drugs prescribed for hyperthyroidism, excessive thyroid function: Thiamazole (Mercazolil), Propylthiouracil.
In this case, the need for a microelement can reach 400 mcg. In any case, the daily intake of I should not exceed the toxic threshold of 500 mcg.
Content
- 1 Name and designation
- 2 History
- 3 Being in nature
- 4 Physical properties 4.1 Isotopes
- 6.1 In medicine
- 7.1 Iodine and the thyroid gland
Causes and signs of deficiency
Iodine deficiency is said to be in cases where its daily intake from food does not exceed 10 mcg. The main reason is the nutritional factor, insufficient intake of I from food due to low content in soil and water. After all, in general it is found in small quantities in the earth’s crust. But this amount is not evenly distributed.
Quite a lot of iodine is dissolved in the waters of the seas and oceans. Therefore, residents of coastal zones almost never suffer from its deficiency - the microelement comes not only from food, but also from inhaled sea air... But the further from the coast inland, and higher into the mountains, the more acutely the deficiency of I is felt.
There are vast regions in Central Africa, Latin America, and Southeast Asia that are endemic for iodine deficiency. But it would be a mistake to believe that this is a problem only in backward countries with low living standards and poor nutrition. Insufficient iodine content has been recorded in certain areas of the USA, Canada, France, Belgium, Spain, Germany, and Poland.
Places endemic for iodine deficiency are found in Belarus and Ukraine. In the Russian Federation, these are vast areas in the central part, as well as in the North Caucasus, Eastern Siberia, and Transbaikalia. And even in Primorye, despite its proximity to the sea, the population suffers from iodine deficiency.
The problem lies not only in natural iodine deficiency, but also in its artificial occurrence due to human activity. As a result of many years of soil exploitation, it will stop even less.
The applied fertilizers do not contain I and do not compensate for its consumption. In addition, soil and water are systematically polluted with pesticides, industrial emissions containing salts of heavy metals, nitrates, and aromatic hydrocarbons.
All these compounds penetrate food and make it difficult to absorb iodine. Another factor is selenium, or rather, its low content. This trace element is necessary for the absorption of iodine. As with iodine, there are regions where selenium deficiency is endemic. This is still the same Siberia, Transbaikalia.
Even with a normal amount of iodine in food, but with a selenium deficiency, iodine will be poorly absorbed. What can we say about the low amount of both microelements, selenium and iodine.
But these are all general reasons. There are also private, individual ones. And here, too, the nutritional factor comes first. Most iodine is found in fish and seafood. Therefore, ignoring these foods, vegetarianism, will lead to insufficient iodine intake.
Chronic gastrointestinal diseases impair the absorption of many food components, and iodine is no exception. Smoking, alcohol abuse, taking medications (antibiotics, anti-inflammatory, antipyretic, some antiarrhythmics) - all this also negatively affects iodine balance.
Some conditions are accompanied by increased iodine consumption:
- physical exercise
- past acute illnesses and injuries
- chronic diseases
- pregnancy
- rapid growth, puberty
- other types of stress loads.
In these cases, relative iodine deficiency occurs. Little iodine means little thyroid hormones. This condition with reduced hormone-producing function of the thyroid gland is called hypothyroidism. In an effort to extract maximum benefit from the available iodine, and at least to some extent increase the production of thyroid hormones, the thyroid gland increases in size.
This enlargement is called a goiter. It should be noted that this is not an independent pathology. The concept of “goiter” is collective. It implies any increase in the size of the gland. The increase can occur evenly (diffuse goiter), and in the form of foci (nodular goiter). At the same time, its hormone-producing function can also vary significantly.
Sometimes a compensatory enlargement of the thyroid gland gives results, and against the background of iodine deficiency, the level of thyroid hormones still returns to normal. This type of goiter is called euthyroid. However, in many cases, despite the increase in size, hypothyroidism persists.
Goiter itself is a physical and mental discomfort. An enlarged gland deforms the neck, and thereby spoils the appearance. In addition, with a significant degree of increase, a mechanical obstruction to breathing is created, which leads to shortness of breath. But women are more susceptible to iodine deficiency. The reason is that the gland is functionally connected to the ovaries and is sensitive to cyclic fluctuations of female sex hormones.
But the main problems are not a cosmetic defect, or even an obstruction to breathing. Although chronic hypoxia in itself is already bad. Lack of iodine and hypothyroidism leads to a slowdown in metabolic processes in the body. Body weight increases due to fat deposition. Obesity, in turn, leads to type II diabetes mellitus, caused by a relative lack of insulin.
The cardiovascular system undergoes negative changes. The strength of myocardial contractions decreases, bradycardia appears, and the heart rate slows to less than 60 beats/min. Congestive heart failure develops and vascular atherosclerosis progresses.
Characteristic appearance of a patient with hypothyroidism. Slowing the release of fluid leads to swelling of the limbs and face, which becomes puffy. The skin becomes dry and pale, the hair becomes brittle. This complex of external changes in hypothyroidism is called myxedema.
Anemia is typical for such patients. Their body temperature is reduced, causing them to complain of chills. Digestion of food worsens. Abdominal bloating, flatulence, and constipation appear. The patients themselves are lethargic and adynamic. Slow thinking, poor memory, and inattention are noted.
In women, the menstrual cycle is disrupted. Hence the problems with conception. The subsequent pregnancy is also accompanied by complications for both the mother and the fetus. The woman quickly gains weight, swelling and unstable blood pressure appear. This is fraught with weakness of labor, eclampsia during childbirth or in the postpartum period. With severe iodine deficiency and hypothyroidism, miscarriages and stillbirths are possible. Some babies are born with developmental defects in the cardiovascular, nervous, and digestive systems.
The bottom line is that the thyroid gland in the fetus begins to function at about 18-19 weeks. intrauterine development. Until this time, the fetus receives maternal thyroid hormones, and their deficiency has a detrimental effect on development. After childbirth, the situation is aggravated by the fact that lactation is difficult, and such children often have to be transferred to artificial feeding.
Children's bodies are especially sensitive to iodine deficiency. Children born and raised in regions where iodine deficiency is endemic often suffer from congenital myxedema or cretinism. This condition is characterized by deviations in physical and mental development.
The pathology affects internal organs, skin, musculoskeletal system, and sensory organs. Dwarfism, disproportionate physique, structural changes in the heart, liver, and kidneys are noted. The skull is deformed, the teeth grow poorly, the face is puffy. Speech, vision, and hearing disorders, including deaf-muteness, are typical. Intellectual impairments range from mild debility to complete idiocy.
All of the above are just signs of severe iodine deficiency and thyroid insufficiency. They are relatively rare. Much more often, iodine deficiency is mild and subclinical. According to WHO research, about 30% of the world's population is deficient in I. Perhaps this is associated with a high incidence of cardiovascular diseases, obesity, and immunological disorders.
Do I need to take iodine-containing medications?
Only a doctor can decide whether a person needs to take iodine. Typically, taking medications containing iodine is recommended:
- during pregnancy planning;
- during pregnancy and breastfeeding;
- newborns whose mothers suffer from iodine deficiency;
- during heavy mental stress (during exams or when working on important projects).
Iodine is one of the most important elements necessary for the normal functioning of the body. However, we must not forget that excess iodine is no less dangerous than its deficiency. It is important for every person to eat right: only a balanced diet will provide the body with everything necessary for its normal functioning. Only a doctor can decide whether a person needs additional medications containing iodine.
Sources of income
UP TO 60% of iodine comes to us in animal products, up to 30% in plant products, and the rest in water. We receive a certain amount of microelement in the inhaled air. The generally recognized leaders in content I are fish and seafood.
Content of I in 100 g of products:
| Product | Content, mg/100 g |
| Dried kelp (sea kale) | 2500-3000 |
| Cooked seaweed | 300 |
| Squid | 290 |
| Shrimp, crab, oysters | 90-100 |
| Pinniped meat | 130 |
| Cod, blue whiting | 130 |
| Salmon | 200 |
| Pollock, hake | 150-160 |
| Perch, pike perch, catfish, pike, flounder, pike perch, capelin | 50 |
| Anchovies, mackerel | 45 |
| Mussels | 190 |
| Champignon | 18 |
| Egg yolk | 35 |
| Milk and dairy products | 8-18 |
| Legumes, vegetables, fresh herbs | 6-15 |
| Berries, fruits, cereals | 2-10 |
| Pine nuts | 400 |
| Hard cheeses | 11 |
During heat treatment, products lose up to 50% or more of the iodine they contain. To minimize the loss of microelements, products should be cooked whole or cut into large pieces over low heat, in a small volume of water, for a short time, and in a container covered with a lid.
Another artificial food source of iodine is iodized salt. This is table salt, sodium chloride, with the addition of iodine compounds - sodium and potassium iodides and iodates. The use of iodized salt is recommended for people living in endemic iodine-deficient areas.
Some facts should be taken into account. Iodized salt gives foods a specific bitter taste, and when heated, the amount of iodine decreases. For these reasons, it should not be used for boiling, cooking, or canning foods. Add it to food immediately before consumption. The shelf life of this salt, compared to regular salt without iodine, is short and is 6 months. It should be stored in a place protected from light.
Foods that contain iodine
If you say “iodine,” you say “sea.” Indeed, it is the depths of the blue ocean that are most rich in this trace element. Therefore, choosing a “marine” food source (sea fish, algae, crustaceans and molluscs) is the best way to provide our body with the appropriate amount of iodine.
Are you not a fan of fish products? Calmly. There are other alternatives. All foods that come from coastal areas are, in fact, rich in iodine. Thus, plants (i.e. vegetables and fruits) and animals (i.e. meat) grown in coastal areas are high in iodine.
However, one clarification must be made: this trace element has a greater tendency to bind to fatty substances, so its concentration depends on the specific characteristics of the food.
Table of iodine in food
| Product | µg/100 g fresh weight (average) | µg/100 g dry weight (average) |
| Freshwater fish | 30 | 116 |
| Sea fish | 832 | 3715 |
| Crustaceans | 798 | 3866 |
| Meat | 50 | — |
| Milk | 47 | |
| Eggs | 93 | |
| Cereals | 47 | 65 |
| Fruits | 18 | 154 |
| Legumes | 30 | 234 |
| Vegetable | 29 | 385 |
| Iodized salt | 30 mg per kg of product |
In any case, WHO, as part of the program for the prevention of iodine deficiency, recommends replacing regular salt with iodized salt, i.e. enriched with iodine.
Synthetic analogues
In most preparations, iodine is present in combination with potassium, in the form of potassium iodide, KI. This compound is included in many medicines.
| A drug | Manufacturer | Release form |
| Iodomarin | Berlin-Chemie, Germany | Tablets 100, 200 mcg. |
| Iodine balance | Merck, Germany | |
| Iodine Vitrum | Unifarm, USA | |
| Potassium iodide | Obolenskoye, Russia | |
| Microiodide | Tatchimpharm, Russia |
These drugs are used to prevent and eliminate hypothyroidism associated with iodine deficiency. They are also indicated for pregnant women to prevent complications of iodine deficiency. But when using them, you cannot exceed the daily dose of 200 mcg. It should be borne in mind that not every hypothyroidism is caused by iodine deficiency.
A decrease in the production of thyroid hormones may be a consequence of congenital anomalies, inflammatory processes in the thyroid gland. Another reason: damage to the pituitary gland and hypothalamus. These brain structures regulate thyroid function. With pathology of the hypothalamic-pituitary system, even against the background of normal iodine content, the production of thyroid hormones will be impaired. Prescribing iodine-containing drugs for all these conditions will be useless.
Another iodine-containing drug is Iodine-Active. Here, iodine is included in the structure of the milk protein casein. Iodaktiv is not a pharmaceutical, but a dietary supplement. Manufacturers claim that it is very difficult to overdose, because... excess iodine is immediately eliminated from the body. But there is no clinical data confirming that this is exactly what happens.
In addition, iodine is included in such complex vitamin-mineral products as Vitrum, Centrum, Vitamax. They are also used to replenish iodine reserves in the body.
But the famous iodine tincture is not suitable for this. But it is an excellent antiseptic. It is an alcohol solution of potassium iodide and crystalline iodine. When applied to a wound surface, it dries it, destroys infection, and indirectly has an anti-inflammatory effect.
But oral administration is contraindicated. Firstly, the alcohol solution irritates the mucous membranes of the mouth, esophagus and stomach. And secondly, in this case it is easy to overdose on iodine. Along with iodine tincture, other iodine-containing antiseptics are also used: Iodonate, Iodopirone, Betadine, Lugol's solution. Some iodine preparations (Yoxilan, Ioversol, Iodixanol, etc.) are used in radiology as contrast agents for the diagnosis of blood vessels, gastrointestinal tract and urinary system.
Metabolism
Iodine absorption occurs in the upper parts of the small intestine, where it enters in the form of organic and inorganic compounds - iodides, iodinated amino acids, and fatty acids. Most of the absorbed iodine binds to a specific carrier protein, and in this form is distributed to organs and tissues.
At the same time, it enters the liver, kidneys, ovaries in women, and the prostate gland in men. A certain amount of the microelement is absorbed by the skin and its appendages, hair and nails.
But the main consumer of I is, of course, the thyroid gland. The functional tissue of this gland consists of many tiny vesicles, follicles. The inside of the follicles is lined with thyrocyte cells and filled with colloid. This viscous substance contains thyroglobulin proteins, which come here from thyrocytes.
The amino acid L-tyrosine is involved in the formation of thyroglobulins. Thyroglobulins are the raw materials for the formation of triiodothyronine and thyroxine.
Iodine first moves from the blood into thyrocytes, and then into the colloid. Here, with the participation of iron and the specific enzyme thyroid peroxidase, sequential iodination occurs, the addition of I atoms to the thyroglobulin molecule.
1 molecule joins - monoiodothyronine is formed, 2 - diiodothyronine. True, these substances are not active. But with the further addition of iodine, triiodothyronine or T3 and thyroxine or T4 are formed.
These substances already have hormonal activity. True, this activity, like their content, is not the same. The content of T4 is several times higher than T3. But T3 is more active than T4. It has been established that T3 is formed from T4 during deiodination, the elimination of an iodine molecule. Thyroid hormones, thyroxine and triiodothyronine, are released into the blood.
In the blood plasma they are bound by another carrier, thyroxine-binding globulin, and in this form are distributed to organs and tissues. Having fulfilled their function, they are partially destroyed and partially removed unchanged. Free iodine, and iodine as part of thyroid hormones, leaves our body mainly in urine, and to a lesser extent in feces.
A certain amount of I is excreted in saliva and sweat. It is noteworthy that the excretion of iodine in the urine (ioduria) can be used to judge the presence of iodine in the body. The more iodine enters the body, the more it is excreted in the urine, and vice versa.
Therefore, low ioduria indicates a lack of I. And for the same reason, determination of iodine in urine is used to identify iodine deficiency conditions.