Ferritin: what is it?
Ferritin is a complex complex of proteins that plays the role of a battery of iron molecules. From the Latin name of the chemical element iron (ferrum, Fe) comes the name of the iron-containing protein complex - ferritin. It is ferritin that supplies iron to every cell that needs it.
The human body does not synthesize iron itself. This element comes to us with food. The level of iron absorbed directly depends on age. The younger a person is, the higher his ability to isolate this element from food and store it in the form of ferritin. Children in the first year of life can absorb the maximum amount of iron – up to 70% of the amount consumed. By the age of 10 years, the body shaves 7 times less of the incoming volume. Adults receive only 10% of the iron they eat per day.
The chemical processes of a healthy person’s body clearly control the amount of iron in the blood and, if necessary, adjust the distribution of the resulting volume of elements. For example, with a sufficient amount of iron in the body, the absorbed part of it can be set aside as a kind of preparation “for a rainy day” - this is ferritin, which, when the first need arises, will deliver iron in the required volume to the organ in need. And with a reduced concentration of iron in the body, ferritin does not replenish its reserves, since the entire absorbed volume is immediately distributed throughout the tissues.
Ferritin is located in the liver, plasma, bone marrow, placenta and spleen. The level of total ferritin in the body is determined by studying the composition of blood plasma.
Prices
| Ferritin | 360 ₽ |
All prices
Name in English: ferritin.
Ferritin is a complex protein complex (iron protein) that serves as the main intracellular iron depot in humans and animals. Structurally, it consists of the protein apoferritin and a ferric atom in the composition of phosphate hydroxide. One ferritin molecule can contain up to 4000 iron atoms. Contained in almost all organs and tissues and is a donor of iron to cells that need it.
In a situation where iron begins to be scarce (frequent blood loss or lack of its supply with food), the human body begins to use its reserve from the tissue. Ferritin levels begin to decline. A long-term lack of iron intake can lead to anemia. Ferritin levels can be reduced long before the symptoms of iron deficiency appear and allow anemia to be diagnosed in time.
Along with this, hemoglobin levels also decrease. The condition is accompanied by insufficient oxygen supply to the cells and tissues of organs throughout the body. The central and peripheral nervous system suffers to a greater extent.
Ferritin: normal levels
Various diseases or certain physiological processes can affect ferritin levels. Natural reasons for decreased performance include:
- periodic female bleeding;
- pregnancy;
- dieting.
To monitor health status as a preventive measure, as well as in case of symptoms typical of iron deficiency, a biochemical blood test for ferritin is performed. The collected biomaterial is examined to understand how many micrograms of iron are contained in one liter of plasma.
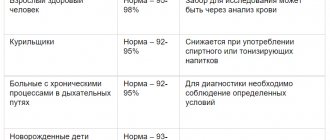
The norms for each age group are different:
- in newborns from 25 to 600 mcg/l;
- in children older than 28 days, but not yet 9 weeks of age, the norm is 20-600 mcg/l;
- in children from 2 to 5 months it should be from 50 to 200 mcg/l;
- children aged from six months to one year should have from 70 to 140 mcg/l;
- girls and women over 12 years of age should have a normal level of 22 to 180 mcg/l;
- boys and men over 12 years old - from 30 to 310 mcg/l.
Women's blood contains less ferritin because it contains less hemoglobin, red blood cells and iron molecules. In addition, pregnancy makes its own adjustments to ferritin levels:
- in the first trimester of pregnancy, levels can reach 90 mcg/l;
- in the second trimester, blood contains up to 74 mcg/l;
- and the third trimester is famous for its low ferritin level - only 10-15 mcg/l.
Iron deficiency anemia during pregnancy - prevention and treatment
M.A. VINOGRADOV
, Ph.D., T.A.
FEDOROVA, Doctor of Medical Sciences, Professor, Scientific Center of Obstetrics, Gynecology and Perinatology named after.
acad. IN AND. Kulakov Ministry of Health of Russia This review is devoted to the problem of prevention and treatment of anemia during pregnancy. Iron deficiency anemia (IDA) is the most common deficiency condition and the most common form of anemia in pregnant women. Its clinical consequences are extremely important, since the adverse effects of iron deficiency affect not only the woman’s body, but can also affect pregnancy outcomes and the health of newborns. The first line of treatment for iron deficiency is iron preparations intended for oral administration, the most effective and safe form of which is currently considered an iron-polymaltose complex. In case of insufficient effectiveness and severe anemia, the preferred alternative method is intravenous iron supplementation. Timely diagnosis and adequate therapy make it possible to restore iron metabolism in a pregnant woman in the shortest possible time and prevent the development of complications.
Introduction
Iron deficiency is known to be the most common nutritional deficiency condition in the world [1] and the most common cause of anemia in pregnant women (up to 75%) [2]. According to the World Health Organization definition, anemia during pregnancy is considered to be a decrease in blood hemoglobin of less than 110 g/l [1], and in the second trimester - less than 105 g/l [3]. It is known that during pregnancy a number of physiological changes occur in a woman’s body, including blood changes. The total plasma volume increases to 50% of the original, and the globular volume increases only by 25% [4, 5]. As a result, the need for microelements and vitamins necessary for the synthesis of hemoglobin and ensuring the normal development of the fetus and placenta increases. In the absence of adequate replenishment of increasing needs, a deficiency of microelements, primarily iron, develops, and, as a consequence, anemia. This is due to many factors: pregnancy often occurs with an initially reduced level of hemoglobin or insufficient iron reserves in the body, which can be caused by diet, chronic diseases of the gastrointestinal tract, or prolonged heavy menstruation. Iron is an essential trace element for humans. During pregnancy, adequate iron status is an important prerequisite for normal fetal development and neonatal maturity. It has been shown that severe anemia with a decrease in hemoglobin less than 90 g/l can contribute to pregnancy complications and adversely affect its outcomes [6]. There is evidence that iron deficiency, even in the absence of iron deficiency anemia (IDA), can also have negative effects on non-pregnant women, for example in relation to cognitive abilities and physical performance. In addition, IDA during pregnancy is associated with a risk of preterm birth and low birth weight [7].
Assessment of iron status in the body
To adequately assess iron metabolism and timely detect iron deficiency, it is necessary to use a number of laboratory tests. In addition to a complete blood count, which provides insight into hemoglobin levels and red blood cell characteristics, iron status can be assessed primarily by testing serum ferritin. Additional tests include transferrin saturation and serum soluble transferrin receptor (sTfR). Serum iron is not a reliable diagnostic parameter, so its study is not enough to clarify the cause of anemia. An isolated hemoglobin test is not suitable for assessing anemia during pregnancy due to the presence of varying degrees of hemodilution in patients [8]. Ferritin provides information about the completeness of iron stores in the body, sTfR and transferrin saturation provide information about the development of iron deficiency at the cellular level, and hemoglobin provides information about iron deficiency at the functional level. For practical purposes, a complete blood count and serum ferritin are sufficient tests to assess iron status and diagnose IDA in most women. It is important that in the presence of an inflammatory process, the ferritin value may be unreliably high. In this case, an increase in C-reactive protein (CRP) is confirmation of an inflammatory process that requires treatment. The diagnostic algorithm for detecting anemia is presented in Figure 1 [9].
Iron requirements during pregnancy
Iron requirements during pregnancy increase from 0.8 mg/day in the first trimester to 7.5 mg/day in the third trimester (average 4.4 mg/day). On average, a normal pregnancy requires an additional approximately 1,240 mg of iron [10, 11]. Studies have shown that many non-pregnant women have reduced iron stores: 42% have serum ferritin less than 30 μg/L, and only 14-20% of them have ferritin levels exceeding 70 μg/L, [12], i.e., iron stores balance the needs of normal pregnancy [10].
We can identify groups of pregnant women who have a higher risk of developing iron deficiency: multiple pregnancies, multiple pregnancies with a short intergravid interval, blood donors, vegetarians, women with low socioeconomic status, patients with chronic diseases of the gastrointestinal tract [13]. It has been proven that there are significant differences between hemoglobin levels in women who receive additional iron during pregnancy and those who do not [14]. In addition, hemodilution causes physiological fluctuations in hemoglobin levels during pregnancy [15]. In women receiving iron supplements during pregnancy, hemoglobin concentrations show a steady decline from the end of the first trimester due to hemodilution, reaching a minimum at 25 weeks of pregnancy. Subsequently, hemoglobin rises during the remainder of pregnancy to reach a peak level shortly before delivery.
Prevention of iron deficiency
Placebo-controlled studies have consistently shown that pregnant women taking iron supplements have significantly higher iron stores compared with women taking placebo [16]. Consequently, women taking iron have a lower incidence of anemia.
Previously, high doses of ferrous iron in the range of 100-200 mg/day were used to prevent iron deficiency in pregnant women. A dose of 100 mg of ferrous iron per day induces a maximum increase in hemoglobin, and 200 mg of iron per day increases ferritin and hemoglobin during childbirth as well as in non-pregnant women. However, the results of the work highlighted the potential negative effects of using such doses of iron [14], and consequently, studies were initiated to study the effectiveness of lower doses of iron, attempting to determine the smallest effective dose [17]. European studies have shown that supplementation with 45–66 mg of ferrous iron per day from 12–20 weeks of pregnancy until delivery is sufficient to prevent IDA in healthy pregnant women. Even lower doses of 20–27 mg of ferrous iron per day have a beneficial effect on iron status [16]. The main conclusions from these studies are, first, that 30–40 mg of ferrous iron per day is sufficient to prevent IDA, and, second, that low-dose iron supplements in the range of 20–27 mg/day are better than no supplementation. whereas studies of daily consumption of multivitamin preparations containing 14–18 mg of ferrous iron showed no effect on iron status in women [13]. Currently, in order to prevent iron deficiency in pregnant women, it is recommended to use an oral iron supplement at a dose of 30-40 mg/day from the beginning of pregnancy until childbirth [14].
Thus, an individualized approach to the prevention of IDA is currently recommended, which is based on the assessment of iron stores (plasma ferritin) before and at the beginning of pregnancy. Women with ferritin levels greater than 70 mcg/l are not advised to take iron supplements; with ferritin values of 30-70 µg/l, 30-40 mg of ferrous iron per day should be taken, and with ferritin levels less than 30 µg/l, 80-100 mg of ferrous iron should be taken per day [14].
Treatment of anemia
If IDA is detected, the deficiency should be corrected with oral or intravenous administration of iron supplements. Intramuscular administration of iron is currently practically not applicable due to the limited dose of iron administered per injection and the high incidence of painful local reactions.
Iron preparations for oral administration
Iron absorption is regulated in accordance with iron reserves in the body and the intensity of erythropoiesis. It has been proven that in case of depletion of iron stores in the body, intestinal absorption of iron increases [18]. In addition, increased erythropoietin-induced erythropoiesis in the second and third trimester [19] stimulates iron absorption.
This combined stimulation of iron absorption has been confirmed in studies showing that with increasing gestational age there is an increase in iron absorption, and this is most pronounced after 20 weeks of pregnancy. The ability to absorb a significant amount of iron through the gastrointestinal tract is favorable for the treatment of IDA with tableted iron supplements in pregnant women. In this regard, the administration of oral iron supplements is first-line therapy, especially during the first and second trimesters of pregnancy [20]. For mild anemia with hemoglobin 90-105 g/l, the recommended dose is 100-200 mg of elemental iron per day. After oral treatment with iron supplements for 2 weeks. the effect should be assessed. If hemoglobin increases by more than 10 g/l, therapy should be continued during the remaining period of pregnancy with subsequent monitoring of hemoglobin and ferritin [13].
Refractoriness of anemia to therapy may be a consequence of non-compliance with the drug due to subjective reasons, gastrointestinal side effects, impaired iron absorption due to achlorhydria or inflammatory bowel disease, or hidden ongoing bleeding with iron loss. Gastrointestinal disorders, such as colic, nausea, vomiting, and diarrhea, occur in approximately 6–12% of patients taking iron supplements [21]. Until recently, iron salts were most widely used internally. However, their use is limited by low and uneven absorption, in particular dependent on food products [22]. Ferric compounds were created to avoid these problems. In the first trimester of pregnancy, the severity of iron deficiency, on the one hand, and a sufficient supply of time, on the other, allow for a smooth correction of iron deficiency conditions using the safest oral iron preparations.
The iron-polymaltose complex was developed as a molecule that dissolves at neutral pH. The drug contains iron in the form of a polymaltose complex of iron (III) hydroxide (PKH), for example Maltofer. This complex is stable and does not release iron in the form of free ions into the intestines. The structure of the drug is similar to ferritin. Due to this similarity, iron (III) moves from the intestine into the blood through active absorption. Iron, which is part of PKZh, does not have the pro-oxidant properties inherent in simple iron salts. Studies to evaluate the effectiveness and safety of polymaltose drugs in comparison with ferrous sulfate in pregnant women have shown their undoubted advantage. Polymaltose iron complex has been formulated so that the elemental form of iron is in a non-ionic state. This ensures that its use does not cause irritation to the gastric mucosa. Additionally, the high iron content eliminates the need for frequent dosing and therefore improves compliance. The polymaltose complex (Maltofer) should be taken during or immediately after meals, which also increases ease of use. Interesting data were obtained in an experiment using iron salts and PLC in pregnant rats. All treatment methods were effective in correcting anemia. However, as a result of the use of iron salts, signs of liver damage and oxidative stress were noted in the study of the fetus and placenta. PLC restored normal expression of TNF-α and IL-6 in the placenta, while the highest levels of cytokines were observed with ferrous sulfate, suggesting a local inflammatory response. Most of the negative effects associated with IDA were resolved by the administration of PKZh. Pregnancy outcomes with the use of iron salts were worse when PCZ was prescribed [23].
The use of various iron preparations in adults has been studied many times. A study by Badhwar et al [24] in both women and men with IDA demonstrated equivalent efficacy and better bioavailability of PCZH compared to ferrous fumarate. However, this study was conducted in non-pregnant women. A study by Pakar et al [25] also demonstrated the effectiveness and safety of PLC in both pregnant and non-pregnant women.
Important results were obtained in a randomized, double-blind, controlled trial of the use of PCZ during pregnancy [26].
The incidence of side effects was significantly higher in the iron salts group than in the PCZ group (78 vs. 31%, p < 0.001). The increased incidence of adverse effects of iron salts may be due to the release of free radicals, leading to cell damage and death [27], whereas PLC does not release free radicals. Reducing the incidence of side effects improves patient compliance and ensures regular treatment. In addition, better tolerability is essential to ensure long-term therapy during pregnancy. PLC is an effective therapeutic approach in the treatment of IDA in pregnant women. The improved tolerability profile compared to iron salt preparations and the equivalent efficacy profile strongly suggest that PKZh is the preferred form of oral iron for the treatment of IDA during pregnancy. Significantly better tolerability of PCZH in comparison with quickly satiating, but less tolerated preparations of inorganic iron salts ensures higher compliance of patients, contributing to the formation of a positive stereotype of daily drug use until the end of pregnancy, which is fundamentally necessary.
Iron preparations for intravenous administration
Treatment with intravenous iron is superior to oral iron in terms of the speed of restoration of hemoglobin and replenishment of iron reserves in the body. However, the safety of such drugs in the first trimester has not been sufficiently proven, so they can be recommended for use from the second or third trimester of pregnancy. Intravenous iron reduces the need for blood transfusions and is an alternative to transfusions in severe IDA. Currently, the most effective therapeutic approach, allowing to safely obtain the maximum effect in the shortest possible time, is the use of iron carboxymaltose [28]. This is a dextran-free complex that can be used in maximum doses (up to 1000 mg per intravenous injection) in a short period of time (15-30 minutes per infusion). Repeated infusions are carried out weekly at a rate of 15 mg of iron per kg of body weight. According to the Cochrane Database [29], among drugs for intravenous use, iron carboxymaltose is the drug of choice for the treatment of IDA during pregnancy. Intravenous iron supplements are considered safe in the second and third trimesters of pregnancy [30], although intravenous iron infusions should be administered by health care personnel to avoid possible allergic or other reactions. Treatment with intravenous iron preparations is indicated in cases of ineffectiveness of iron-containing drugs for oral administration (no increase in hemoglobin by 10 g/l within 2 weeks), with severe IDA (hemoglobin <90 g/l during pregnancy more than 14 weeks, and also as first-line treatment for IDA in the third trimester. At this stage there is insufficient time for oral therapy to be effective. This is important to reduce the manifestations of IDA and replenish iron stores before delivery to prevent anemia in labor and avoid blood transfusions The dose of intravenous iron should be sufficient to achieve a hemoglobin level of more than 105 g/l. For most women, a total dose of 1,000-1,250 mg of intravenous iron is adequate. When the hemoglobin level reaches 105 g/l, a transfer to maintenance therapy with an oral iron preparation is carried out 100 mg/day until the end of pregnancy.
Conclusion
Iron deficiency anemia is the most common form of anemia in pregnant women (up to 95%). The diagnosis of IDA is based on identifying a decrease in blood hemoglobin and serum ferritin levels. Among non-pregnant women of reproductive age, up to 40% have insufficient iron reserves in relation to the upcoming pregnancy, therefore, issues of timely correction of iron deficiency before the development of anemia are extremely important when planning pregnancy. For this purpose, complex preparations are used containing from 30 to 80 mg of iron, depending on the values of serum ferritin. Given the increasing iron requirements during pregnancy, dietary measures are insufficient to correct iron deficiency. Treatment of IDA during pregnancy should be carried out using drugs for oral administration, the most preferred of which is polymaltose complex of iron (III) hydroxide and iron preparations for intravenous administration. For IDA with a hemoglobin level of more than 90 g/l, the first line of therapy is PKH at a dose of 100-200 mg/day. Indications for prescribing an intravenous iron supplement are anemia with a hemoglobin level of less than 90 g/l, insufficient effect of therapy with oral medications (hemoglobin less than 100 g/l for 2 weeks) or poor tolerability. Timely detection and effective therapy, safe for pregnant women, allows you to quickly normalize hemoglobin levels and improve iron reserves, which, in turn, improves the quality of life of women and prevents the development of pregnancy complications. Taking into account the inevitability of the development of one or another degree of iron deficiency during gestation in the vast majority of pregnant women and taking into account the negative long-term consequences of iron deficiency on the ante- and postnatal development of the child, the tactics of early initiation of anemia treatment should be considered the most justified.
Literature
1. WHO Iron Deficiency Anaemia: Assessment, Prevention and Control. WHO/NHD/01.3, World Health Organization, 2001, Geneva, Switzerland. 2. Sifakis S and Pharmakides G. Anemia in pregnancy. Ann. NY Acad. Sci., 2000, 900: 125–36. 3. Ramsey M, James D & Steer P. Normal Values in Pregnancy, 2nd edn. WB Saunders, London, 2000. 4. Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Current opinion in clinical nutrition and metabolic care. 2011, 14, 625-634. 5. Friedman AJ et al. Iron deficiency anemia in women across the life span. Journal of women's health, 2012, 21: 1282-1289. 6. The Obstetric Hematology Manual edited by Sue Pavord, Beverley Hunt. Cambridge University Press 2010. pp. 13-27. 7. Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z (2007). Low first trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet 98: 124–128. 8. Koller O (1982). The clinical significance of hemodilution during pregnancy. Obstet Gynecol Surv 37:649–652. 9. Breymann Ch et al. Diagnosis and treatment of iron-deficiency anemia during pregnancy and postpartum. Arch Gynecol Obstet 2010, 282:577-580. 10. Milman N (2006). Iron and pregnancy - a delicate balance. Ann Hematol 85:559–565. 11. Bothwell TH (2000). Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 72:257–264. 12. Milman N, Byg KE, Ovesen L (2000). Iron status in Danes updated 1994. II. Prevalence of iron deficiency and iron overload on 1319 women aged 40–70 years. Influence of blood donation, alcohol intake, and iron supplementation. Ann Hematol 79: 612–621. 13. Milman N. Prepartum anemia: prevention and treatment. Ann Hematol 2008, 87:949–959. 14. Milman N (2006). Iron prophylaxis in pregnancy—general or individual and in which dose? Ann Hematol, 85: 821–828 doi:10.1007/s00277-006-0145-x. 15. Milman N, Bergholdt T, Byg KE, Eriksen L, Hvas AM (2007). Reference intervals for haematological variables during normal pregnancy and postpartum in 433 healthy Danish women. Eur J Haematol 79: 39–46. 16. Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM (2003). Efficacy and tolerance of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr 78: 145–153. 17. Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, Hertz J (2005). Iron prophylaxis during pregnancy — how much iron is needed? A randomized, controlled study of 20 to 80 mg ferrous iron daily to pregnant women. Acta Obstet Gynecol Scand 84: 238–247. 18. Skikne B, Baynes RD (1994). Iron absorption. In: Brock JH, Halliday JW, Pippard MJ, Powell LW (eds) Iron metabolism in health and disease. Saunders, London, pp. 151–187. 19. Milman N, Graudal N, Nielsen OJ, Agger AO (1997). Serum erythropoietin during normal pregnancy: relationship to hemoglobin and iron status markers and impact of iron supplementation in a longitudinal, placebo-controlled study on 118 women. Int J Hematol 66: 159–168. 20. Beris P, Maniatis A on behalf of the NATA working group on intravenous iron therapy (2007). Guidelines on intravenous iron supplementation in surgery and obstetrics/gynecology. TATM transfus Altern Transfus Med 9(Suppl 1): 29. 21. Adamson JW. Fauci AS. Kasper DL, et al. Iron deficiency and other hypoproliferative anaemias. In: Braunwald E, editor; Harrison's Principles of Internal Medicine.15th edition. Mc Graw Hill; 2001. pp. 660–66. 22. Sharma N. Iron absorption: IPC therapy is superior to conventional iron salts. Obstet Gynecol., 2001: 515–19. 23. Toblli JE, Cao G, Oliveri L, Angerosa M. Effects of iron polymaltose complex, ferrous fumarate and ferrous sulfate treatments in anemic pregnant rats, their fetuses and placentas. Inflamm Allergy Drug Targets, 2013, 12(3): 190-8. 24. Badhwar VR, Rao S, Fonseca MM. Comparative efficacy and safety of iron polymaltose+folic acid and oral ferrous fumarate in the treatment of adult patients with iron deficiency anemia. Indian Med Gazette 2003, 136: 296–301. 25. Patkar VD, Patkar S, Khandeparker PS, Dingankar NS, Shetty RS. Evaluation of efficacy and tolerability of iron (III) – hydroxide polymaltose complex tablets in the treatment of iron deficiency anemia in women. Indian Med Gazette 2001, 135: 306–309. 26. Saha L, Pandhi P, Gopalan S, Malhotra S and Saha PK. Comparison of Efficacy, Tolerability, and Cost of Iron Polymaltose Complex With Ferrous Sulphate in the Treatment of Iron Deficiency Anemia in Pregnant Women. MedGenMed, 2007, 9(1): 1. 27. McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol., 1998, 35: 5–12. 28. Christoph P, Schuller C, Studer H et al. Intravenous iron treatment in pregnancy: comparison of high-dose ferric carboxymaltose vs. iron sucrose. J Perinat Med, 2012, 13, 40(5), 469-474. 29. Reveiz L, Gyte GML, Cuervo LG, Casasbuenas A. Treatments for iron-deficiency anemia in pregnancy (Review), Cochrane library, 2011. 30. Beris P, Maniatis A, on behalf of the NATA working group on intravenous iron therapy (2007) Guidelines on intravenous iron supplementation in surgery and obstetrics/gynecology. TATM transfus Altern Transfus Med 9(Suppl 1):29.
Source:
Medical Council, No. 9, 2015
Why is low ferritin dangerous?
A decrease in ferritin levels is primarily an indication for analysis of the nutritional system. Most often, insufficient iron levels in the blood are caused by improper diets or fasting. The second most popular cause is bleeding and diseases of the digestive system. With blood loss, large volumes of iron leave the body, and due to impaired absorption function, the deficiency cannot be replenished.
With a lack of ferritin, the following symptoms appear:
- brittle nails;
- hair loss;
- decreased libido;
- mood swings;
- increased heart rate;
- dizziness;
- memory impairment.
Low ferritin, if not detected in time, and nutrition adjustments and the necessary treatment are not started, can become an impetus for the development of iron deficiency anemia.
Indications for the purpose of the study
- Hereditary hemochromatosis. In this disease, too much iron is absorbed from food and deposited in various organs, causing them to become damaged.
- Multiple blood transfusions, intramuscular iron administration, administration of iron tablets.
- Inflammations, such as upper respiratory tract infections, urinary tract infections, autoimmune diseases. Moreover, an increase in ferritin in the acute phase of inflammation can mask the existing iron deficiency.
- Acute or chronic liver diseases.
- Alcoholism.
- Hemolytic anemia: associated with the destruction of red blood cells, B12 deficiency anemia, thalassemia.
- Hyperthyroidism is an increase in thyroid function.
- Oncological diseases of the bone marrow, breast cancer, Hodgkin's disease - a malignant neoplasm of lymphoid tissue. Ferritin levels will increase significantly.
High ferritin levels
High ferritin, close to the normal limit, indicates that tissues and organs receive a sufficient amount of iron every day from the diet, thanks to which the body is able to store reserves of an important element. If the values obtained as a result of laboratory tests gave inflated indicators, significantly beyond the acceptable limits, this is a reason to examine to identify:
- oncology;
- infection with the immunodeficiency virus;
- problems in the functioning of the endocrine system.
Separately, you need to analyze your diet. Perhaps excess iron can easily be explained by a diet rich in this element.