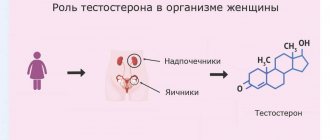
What is the hormone estrogen? The definition states that estrogens are a group of female hormones that are also found in small quantities in male bodies. There are many varieties of these hormones in the body, the main ones being estradiol, estriol and estrone.
What do the predominant three types of these hormones affect in women?
- Estradiol is the most active. It is administered by injection to women with severe hormonal deficiency. Estradiol is the basis of most combined oral contraceptives.
- Estrone - stimulates the development of the uterus and the growth of the mucous membrane in its cavity, as well as the formation of secondary sexual characteristics.
- Estriol - formed from the first two types. If its amount in the urine of a pregnant woman is increased, this indicates that the placenta is working well and the fetus is viable.
In women, in the first half of the menstrual cycle, these hormones are formed in the follicle, and in the second half - in the corpus luteum. Female estrogens are produced in smaller quantities by the adrenal glands. After menopause, their production occurs in the adrenal cortex. In men, these hormones are produced mainly in the testicles.
Estrogen production increases significantly during pregnancy. During this period, they are produced in the placenta.
Why are estrogens needed?
If the female body contains enough estrogen, the “symptoms” that first catch the eye are a beautiful figure with a thin waist and rounded hips, as well as velvety skin.
Estrogens in women are steroid hormones: they affect the growth and development of the genital organs, preparing the woman for motherhood. Under their influence, the following changes occur in the body:
- secondary sexual characteristics are formed (hair appears in the armpits and pubic area, the characteristic shape of the pelvis is formed, and the mammary glands enlarge);
- an acidic environment is created in the vagina (for the period from puberty to menopause);
- the size of the uterus increases;
- fat cells are distributed (on the chest, hips, buttocks, knees), which gives the figure a more feminine outline.
If the female body contains this hormone in sufficient quantities, symptoms appear in regular periods.
How to normalize hormonal levels?
How to treat excess estrogen in women? In order to improve hormonal levels and eliminate high levels of estrogen in women and symptoms, you will have to seriously take care of your health. Doctors recommend getting a good night's sleep, walking a lot in the fresh air, and trying not to get nervous. A good way to prevent hormonal imbalances is to exercise. In addition, it is worth paying attention to nutrition. Estrogen production is stimulated by soy, cheeses, yogurts, legumes, coffee and cabbage.
You can make an appointment with a specialist who, after examination and tests, will prescribe effective treatment now by following the link https://45plus.rf/registration/. A qualified specialist will analyze in detail excess estrogen in women, treatment, symptoms, and prescribe a number of recommendations or medications that are most suitable for you, taking into account all the characteristics of the body.
Increased hormone levels
Among women
Some of the most important hormones in a woman’s body are estrogen and progesterone. Both are produced in the ovaries. These hormones are released at different phases of the menstrual cycle.
On the first day of menstruation, the pituitary gland releases follicle-stimulating hormone (FSH). Under its influence, the follicle begins to develop on the ovary and estrogens are released. As soon as their level rises to a certain point, they block the release of FSH. This occurs approximately on the 12th–15th day of the cycle.
At this time, the pituitary gland begins to secrete another hormone - luteinizing hormone (LH). When its level rises, ovulation occurs - the follicle bursts and an egg ready for fertilization emerges. After ovulation, estrogen levels decrease and progesterone begins to be produced in the ovaries.
Schematically, the production of hormones during the menstrual cycle can be represented as follows:
follicle-stimulating hormone (pituitary gland) → estrogens (ovaries) → luteinizing hormone (pituitary gland) → progesterone (ovaries)
Oral contraceptives operate on this principle: they regulate the amount of female hormones in the body. At the same time, they reduce the level of FSH to such an amount that luteinizing hormone does not begin to be produced. No luteinizing hormone - no ovulation.
In men
Normally, in men, the amount of estradiol should fluctuate between 50–130 pmol/l. Increased hormone levels in men may indicate a tumor in the testicles.
ESTROGEN METABOLISM IN WOMEN
(general concepts and clinical practice)
PART II
ESTROGEN METABOLISM IN WOMEN (GENERAL VIEWS AND CLINICAL PRACTICE).
PART 2. TREATMENT OF HYPERESTROGENEMIA
Estrogen dominance is a fairly common disorder of the metabolism of female sex hormones and is manifested by the development of the following diseases in women: endometriosis, endometrial polyps, cervical dysplasia, fibrocystic mastopathy, uterine fibroids, etc. The presence of the listed diseases in the patient indicates a violation of estrogen metabolism, and the degree of metabolic imbalance is beyond the compensatory capabilities of the body. These diseases cannot be tolerated, since aggravation of estrogen metabolism disorders can lead to the development of malignant neoplasms of the uterus and mammary gland. In our opinion, it is undesirable to use synthetic hormones to treat these diseases. This is due to the fact that the metabolism of synthetic hormones mainly occurs through the genotoxic pathway and, in addition, they can block key enzymes that detoxify female sex hormones. Thus, according to research, the metabolites of the synthetic hormones equilin and equivelin are converted in the liver into 4-hydroxy-o-quinone-glutathione conjugates. The saturated beta ring of these hormones switches the synthesis of 2-hydroxy metabolites of female sex hormones to the 4-hydroxy pathway. [1]. In addition, endogenous 4-hydroxyestrone has a weaker effect on inducing DNA damage and apoptosis in breast cancer cell cultures compared to 4-hydroxyequilenine [2]. The influence of metabolites of synthetic hormones on the activity of key detoxification enzymes is evidenced by the following data. Thus, 4-hydroxyequilenine is an irreversible inhibitor of COMT, which catalyzes the methylation of 4-hydroxyestradiol [3]. 4-hydroxy-equilenin, as well as 4,17-hydroxyequilenin. significantly reduces the activity of glutathione-S-transferase literally a few MINUTES after administration into the body [4].
1 CAUSES OF DISEASES
The primary task of the doctor before starting treatment is to try to find the reasons that led to the development of these diseases.
Here is a far from complete list: smoking, taking hormonal drugs, chronic stress, poor-quality and unbalanced nutrition, exposure to toxic substances at work and at home (phthalates, pesticides, heavy metals, etc.), excessive physical activity, insufficient intake of vitamins , minerals, essential fatty acids, antioxidants, associated diseases, in particular diseases of the digestive system, endocrine organs, obesity, etc.
I would like to pay special attention to the constant or periodic use of pharmaceuticals. It turns out that a number of medications have a rather negative effect on the exchange of female sex hormones. So. a drug that is widely used to treat peptic ulcers and diseases associated with high acidity - omeprazole. is a powerful stimulator of cytochrome P-450 1B1, the activation of which leads to the formation of more converging estrogen metabolite - 4-hydroxy-estrone - in a woman’s body. Phenobarbital and other barbitols:. hypoglycemic drugs (troglitaeon, pioglitazone), anti-tuberculosis drugs (rifampicin) strongly activate the work of another enzyme - cytochrome ZA4, which converts estrone into 16-hydroxyestrone. The latter is a very active metabolite of female sex hormones, increased concentrations of which are associated with long-term risk of uterine and breast cancer.
2 TREATMENT. MAIN DIRECTIONS FOR CORRECTION OF ESTROGEN METABOLISM
The main target of treatment in the case of estrogen dominance is the correction of the metabolism of female sex hormones. Such tactics will not only successfully cure the disease, but also eliminate the possibility of relapse of the disease.
1. Stimulation of the preferential (2-hydroxy-) detoxification pathway of female sex hormones
Very often, patients with hyperestrogenemia have low levels of 2-hydroxyestrone, a “good” metabolite of female sex hormones, which indicates a reduced activity of this hormone conversion pathway. To stimulate it, first of all, it is necessary: to halve the number of cigarettes smoked (benzopyrene, a toxin that is formed as a result of smoking, is destroyed along the same path). In the diet, it is necessary to increase the consumption of raw cruciferous vegetables (cabbage, Brussels sprouts, broccoli, watercress, cauliflower), and also prescribe the following drugs: indole-3-carbinol (I3C), soy isoflavones, omega-3 fatty acids.
A. Indole-3-carbinol (I3C)
Indole-3-carbinol (I3C) is a powerful activator of cytochrome P450 1A1, which converts estrogens into 2-hydroxy metabolites. This substance is obtained from cruciferous vegetables and is very unstable. Therefore, high-quality drugs always have a capsule form, to which an antioxidant is added for stabilization, and usually their shelf life does not exceed 1 year from the date of production. The dose of I3C should be 300-450 mg per day. The duration of treatment is at least 3 months and is determined based on repeated laboratory tests. Restoration of normal concentrations of 2-hydroxyestrogens and the ratio of 2-hydroxy- to 16-hydroxyestrogens is grounds for discontinuation of therapy or transfer to a maintenance dose.
Due to the powerful activation of cytochrome P450 1A1 in a woman’s body, the concentration of 2-hydroxyestrogens, the most preferred metabolites in the period before menopause, increases [5].
In addition to stimulating 2-hydroxylation, I3C has many different effects on cells in the body. Thus, I3C was found to stimulate apoptosis of precancerous and cancerous breast cells, and this effect is associated with the fact that I3C blocked the activation of NF-kappa-B caused by transfection of the Akt gene. Thanks to this effect, the authors of the article concluded that I3C has a preventive effect against certain forms of breast cancer [6]. The data obtained are consistent with the results of other studies that showed that I3C induces apoptosis of breast cancer cells independently of the p53 and Bax genes [7]. Similar data were obtained in studies of prostate cancer cells. I3C stimulated apoptosis of these cells by reducing NF-kappa-B activation and inhibited their growth by inhibiting the GT phase of the cell cycle [8]. A study of the effect of I3C on cervical cells showed that it inhibited the anti-apoptotic effect of estradiol on epithelial cells exposed to potential mutagenic substances [9].
A study of the effect of I3C on human colon epithelial cell lines showed that this substance has an anticancer effect due to STIMULATION of apoptosis and enhancing the detoxification capabilities of cells in response to exposure to potential mutagenic substances [10]. These data are consistent with studies showing that I3C reduces the risk of colon cancer and polyps in animal models [11].
The anticancer effect of I3C is not only due to its effect on estrogen metabolism. Thus, it was found that I3C blocks angiogenesis in tumor tissue [12]. influences carcinogenesis due to the binding of free radicals [13]. I3C also increases the activity of glutathione D-transferase and quinone reductase. This leads to a decrease in the concentration of quinones and semiquinones, carcinogenic metabolites of female sex hormones [14]. Induction of detoxification enzymes of the second phase of detoxification, such as glutathione-B-transferase, increases the protection of body cells from compounds that have a mutagenic effect. Thus, it was found that I3C induces the activity of at least 11 different enzymes of the second phase of detoxification [15].
Thus, analyzing the above scientific data, it can be argued that I3C stimulates the 2-hydroxy pathway - the preferred pathway for detoxification of female sex hormones, has a modulating effect on estrogen receptors, stimulates the apoptosis of cancer cells, has antioxidant properties and stimulates enzymes of both the former and the second phase of detoxification in the body.
B. Soy isoflavones
In the case of hyperestrogenemia, we obtained a positive effect from the use of soy isoflavones. There are several mechanisms of their therapeutic action. First of all, soy isoflavones stimulate the 2-hydroxylation of estrogens and thus increase the ratio of 2- to 16-hydroxyestrogens. Some authors consider this effect of soy isoflavones in the context of their anticancer effects [16]. Consumption of soy isoflavones at a dose of 65–132 mg per day reduced the excretion of 4-hydroxyestrone, a potentially carcinogenic estrogen metabolite in menopausal women [17]. Similar data were obtained when studying the effect of soy isoflavones on the metabolism of female sex hormones in the period before menopause [18]. Another beneficial effect of soy isoflavones is their ability to induce the synthesis of sex hormone binding protein in the blood serum. Increasing its concentration reduces the bioavailability of estradiol for estrogen-sensitive tissues [19].
The therapeutic dose of soy isoflavones should be 90-180 mg per day for several months. Discontinuation or transfer to a maintenance dose of 45-90 mg is carried out only after achieving normal levels of estrogens and their metabolites and subject to clinical regression of the diseases.
It should be remembered that soy protein may not be a good source of isoflavones, as they are lost during industrial processing of soybeans.
B. Other factors affecting estrogen metabolism
It turned out that consumption of omega-3 fatty acids stimulates the 2-hydroxylation of estrogens. However, the mechanism of action of omega-3 fats remains unknown.
A study of the level of physical activity in women showed that dosed physical activity increases the ratio of 2- to 16-hydroxyestrogens [20].
2. Inhibition of undesirable (16-hydroxy- and 4-hydroxy-) metabolic pathways of female sex hormones
As mentioned earlier, the 16- and 4-hydroxy pathways of female sex hormone metabolism are less desirable in premenopausal women. Therefore, reducing the activity of enzymes that are involved in the metabolism of estrogens along these pathways is a fairly important therapeutic goal.
A decrease in the activity of cytochrome P450 3A4 will lead to a decrease in the formation of 16-hydroxyestrone, a metabolite that is 8 times more active than estradiol and thus significantly weakens its effect on estrogen receptors. The most powerful inhibitor of cytochrome P450 3A4 activity is naringenin, a flavonoid found in grapefruits. According to some studies, one glass of grapefruit juice reduces the activity of cytochrome P450 3A4 by 30% within 12 hours [21]. Other natural inhibitors of this enzyme activity include licorice root, garlic and St. John's wort.
Reducing the activity of cytochrome P450 1B1 is possible with the help of isoflavones found in the kyuju plant (Pueraria lobata). The most active isoflavone of this plant is puerarin, which has the ability to inhibit the activity of some cytochrome P450s. It was found that it inhibits the activity of cytochromes 3A, 2E1 and 2B1, and therefore can reduce the formation of 4-hydroxyestrogens. In addition, it stimulates the activity of cytochrome 1A2, which causes an increase in the production of 2-hydroxyestrogens [22].
3. Modulation of the function of female sex hormone receptors
A. Soy isoflavones
Soy isoflavones have the ability to modulate estrogen receptors.
In vitro and in vivo studies have shown that estrogens have a weak estrogenic effect, which is approximately 1000 times weaker than the effect of estradiol [23]. Another study showed that isoflavones, by binding to estrogen receptors, can compete with estradiol for the receptor or even block receptor function [24].
B. Indole-3-carbinol (I3C)
It has been shown that a product of I3C metabolism in the body can act as a ligand for estrogen receptors and exhibit tamoxifen-like effects on estrogen-responsive tissues [25].
Other studies have shown that 13C is an antagonist of o-estrogen receptors. In addition, this substance, on the one hand, suppresses the estrogen-sensitive genes pS2 and cathepsin-D, and on the other, increases the activity of the BRCA1 gene, which, in turn, blocks the transcriptional activity of estrogen receptor alpha [26].
B. Naringenin and resveratrol
Naringenin, a flavonoid found in grapefruits, and the antioxidant resveratrol, found in red grapes, influence estrogen signaling through direct and indirect blocking effects on estrogen receptor α [27]. Moreover, the mechanism of action of these nutrients is different [28].
D. Vitamin B6
Pyridoxine (vitamin B6) affects the transmission of signals of steroid hormones, including estrogens. Studies have shown that vitamin B6 interacts with the hormone-receptor complex and reduces its binding to DNA and thus disrupts the transmission of the hormonal signal to cell DNA [29]. Based on this, patients with high estrogen levels should receive fairly high doses of vitamin B6 (30-50 mg per day).
4. Combat oxidative stress
Oxidative stress leads to the formation of semiquinones and quinones - metabolites of female sex hormones that cause irreversible damage to cell DNA. To combat oxidative stress, you need to do two things. First, provide the necessary nutrients to your own antioxidant system. So. Copper and zinc are required for superoxide dismllase. selenium and acetylcysteine - for glutathione peroxidase. vitamin B3 - for glutathione reductase. iron - for catalase. Secondly, prescribe plant antioxidants, such as green tea catechins, lycopene, turmeric, limonene, vitamins C and E, and β-carotene, which can inactivate reactive oxygen species.
5. Maintenance of enzymes of the second phase of detoxification, which take part in the final inactivation of female sex hormones
Treatment measures to maintain the final elimination of female sex hormones should cover 3 main pathways of the second phase of detoxification.
A). An important condition for the normal functioning of methylation processes in the body is the consumption of sufficient amounts of protein. This is due to the fact that the universal donor of methyl groups in the body is s-adenosine-methionine, which, in turn, is formed from the amino acid methionine, consumed mainly with animal protein. Therefore, it is necessary to conduct a comprehensive assessment of a woman’s nutrition and, in case of insufficient protein intake, introduce protein foods into the diet. To accurately assess protein needs, it is better to use special computer programs that allow not only to correctly assess the quality of a woman’s diet, but also to restore the detected imbalance. These programs can be found on the website www.anti-aging.kiev.ua.
In addition to protein, methylation processes require micronutrients, which are cofactors of enzymatic methylation systems.' Therefore, the treatment plan should include the following vitamins and minerals:
- folic acid in a dose of 400-1000 mg per day;
- vitamin B6 in a dose of 10-40 mg per day;
- vitamin B2 in a dose of 15-30 mg per day;
- vitamin B12 at a dose of 250-500 mcg per day;
- magnesium in a dose of 400-800 mg per day.
However, the above therapy may not be effective in all patients. This is due to the fact that approximately 25% of Europeans have a genetic polymorphism in MTHFR, a key enzyme in methylation processes in the body. In these patients, methylation processes can be restored either using the methylated form of folic acid (5-methyl-tetrahydrofolate), or using betaine hydrochloride, which acts as a donor of methyl groups, regardless of 5-methyl-tetrahydrofolate. Thus, if the genetic characteristics of the patient are not known, it is better to prescribe a B vitamin complex containing the methylated form of folic acid and betaine hydrochloride.
B). Conjugation with glutathione is involved in the inactivation of quinones, genotoxic metabolites of female sex hormones. For the normal occurrence of this reaction, N-acetylcysteine is necessary, an amino acid that limits the synthesis of glutathione. It has been shown that the administration of this amino acid leads to a significant increase in the concentration of reduced glutathione in tissues and acceleration of conjugating reactions with glutathione [30]. Silymarin also stimulates endogenous glutathione synthesis [31].
IN). To maintain sulfitation activity, molybdenum and inorganic sulfates should be prescribed.
G). Glucose is required to activate glucuronidation processes. Therefore, absolute insulin deficiency or insulin resistance, which are accompanied by a decrease in glucose concentration inside insulin-dependent cells, are always accompanied to some extent by disturbances in glucuronidation. In addition to carbohydrate metabolism, the activity of glucuronidation processes is actively influenced by triiodothyronine, the most active hormone of the thyroid gland. T3 stimulates the expression of UDP-glucuronosyltransferase genes, and this activation depends on the body’s supply of vitamin A [32]. Along with T3, glucosonilates, present in watercress and other cruciferous vegetables, are powerful inducers of the key glucuronidation enzyme, UDP-glucoronosyltransferase [33]. Artichoke extract also has a similar effect [34].
6. Prevention of enterohepatic recirculation of female sex hormones
To prevent the absorption of female sex hormones in the intestines, first of all, it is necessary to consume a sufficient amount of fiber. Women who follow this recommendation have lower levels of estrogen in their blood [35]. Soy fiber and flax seed fiber absorb estrogens most actively. A major role in the recycling of estrogens from the intestine is played by 3-glucuronidase, a bacterial enzyme that cleaves glucuronic acid from detoxified compounds (estrogens). The most powerful inhibitor of the activity of this enzyme is calcium glucarate, a nutrient present in some vegetables [36]. In addition, Silymarin and licorice root also reduce its activity [37].
7. Reduced aromatase activity
As mentioned earlier, aromatase is an enzyme that converts andorostenedione to estrone and testosterone to estradiol. However, in some cases (obesity, exposure to herbicides [38], exposure to pesticides [39, 40], excessive alcohol consumption [42]) the activity of this enzyme increases significantly, which leads to the formation of large amounts of estrogens. High enzyme activity is indicated by normal or decreasing levels of androstenedione and testosterone in the peripheral blood, accompanied by high concentrations of estradiol and estrone. Today there are a fairly large number of aromatase inhibitors used to treat breast cancer. Among these drugs are Anastrasol and Arimidex. In addition, there are a number of natural compounds that have the ability to reduce enzyme activity. Among them, chrysin, apigenin, and naringenin can be distinguished [41]. However, aromatase inhibitors must be used with caution, as their use may cause relative or absolute androgen dominance. This will lead to acne, especially just before or during menstruation, ovarian cysts, and other symptoms of androgen dominance.
3 CONCLUSION
Thus, in the treatment of diseases associated with estrogen dominance, a safe and highly effective alternative to hormone therapy and therapy aimed at inhibiting the secretion of gonadotropins has emerged, which has a fairly stable positive effect on the course of the disease. The good clinical results of this approach are due to the impact of treatment on the causes that led to estrogen dominance, an understanding of the entire complex chain of estrogen metabolism, an integrated approach to the correction of hormonal imbalances, and the use of safe natural compounds that have a powerful modulating effect on estrogen metabolism.
BIBLIOGRAPHY
- Zhang F, Chen Y, Pis ha E, Shen L, Xiong Y, van Breemen KB, Bolton JL. The major metabolite of equilin, 4-hydroxyequilin, autoxidizes to an o-quinone which isomerizes to the potent cytotoxin 4-hydroxyequiknin-o-quinone. Zhang E, Chem Res Toxicol 1999 12(2):204-213.
- Chen Y, Liu X, Pisha E, Constantinou AI, Hua Y, Shen L, van Breemen RB, Elguindi EC, Blond SY, Zhang F, Bolton JL. A metabolite of equine estrogens, 4-hydroxyequilenin, induces DNA damage and apoptosis in breast cancer cell lines. Chem Res Toxicol (United States) May 2000, 13(5):342-350.
- Yao J, Li Y, Chang M, Wu H, Yang X Goodman JE, Liu X Liu H Mesecar AD, Van Breemen RB, Yager JD, Bolton JL. Catechol estrogen 4-hydroxyequilenin is a substrate and an inhibitor of catechol-O-methyltransferase. Chem Res Toxicol (United States) May 2003;16(5):668-675.
- Yao J, Chang M, Li Y, Pisha E, LiuX, Yao D, Elguindi EC, Blond SY, Bolton JL. Inhibition of cellular enzymes by equine catechol estrogens in human breast cancer cells: specificity for glutathioneS-transferase Pl-1. Chem Res Toxicol (United States) Jul 2002;15(7):935-942.
- Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst May 21 1997;89(10):718-723.
- Rahman KM, Li Y, Sarhar FH. Inactivation of akt and NF-kappaB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr Cancer 2004;48(l):84-94.
- Ge X Fares FA, Yannai S. Induction of apoptosis in MCF-7 cells by indole-3-carbinol is independent of p53 and bax. Anticancer Res, Jul-Aug 1999; 19(4B):3199-3203.
- Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarhar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, Gl cell cycle arrest and apoptosis in prostate cancer cells. Oncogene May 24 2001;20(23):2927-2936.
- Chen D, Carter TH, Auborn KJ. Apoptosis in cervical cancer cells: implications for adjunct anti-estrogen therapy for cervical cancer. Anticancer Res Sep-Oct 2004;24(5A):2649-2656.
- Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res (United States) Aug 15 2001;61(16):6120-6130.
- Kim DJ Shin DH, Ahn B, KangJS, Nam KT, Park CB, Kim CK, HongJT, Kim YB, Yun YWJangDD, YangKH. Chemoprevention of colon cancer by Korean food plant components. Mutat Res Feb-Mar 2003;523-524:99-107.
- Chang X, Toi JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis Apr 2005;26(4):771-778.
- ArnaoMB, Sanchez-Bravo J, Acosta M. Indole-3-carbinol as a scavenger of free radicals. Biochem Mol Biol Int (Australia) Aug 1996;39(6): 1125-1134.
- Staack R,Kingston S] Wallig MA, Jeffery EH. A comparison of the individual and collective effects of four glucosinolate breakdown products from Brussels sprouts on induction of detoxification enzymes. Toxicol Appl Pharmacol Mar 1998;149(l):17-23.
- Wallig MA, Kingston S, Staack R, Jefferey EH. Induction of rat pancreatic glutathione S-transferase and quinone reductase activities by a mixture of glucosinolate breakdown derivatives found in Brussels sprouts. Food Chem Toxicol (England) May 1998;36(5):365-373.
- Kishida T, Beppu M, Nashiki K Izumi T, Ebihara K Effect of dietary soy isoflavone aglycones on the urinary 16alpha-to-2-hydroxyestrone ratio in SZN/HeJ mice. Nutr Cancer (United States) 2000;38(2):209-214.
- Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev Aug 2000;9(8):781-786.
- XuX Duncan AM, Merz BE, Kurzer MS. Effects of soy iwflavones on estrogen and phytoestrogen metabolism in premenopausal women. Effects of soy isoflavones cm estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev Dec 1998;7(12):1101-1108.
- Adlercreutz H, Hockerstend K, Bannwart, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone-binding globulin (SHBG). J Steroid Biochem 1987;2 7(4-6):1135-1144.
- Matthews CE, Fowke JH, Dai Q, Leon Bradlow H, Jin F, Shu XO, Gao YT, Longcope C, Hebert JR Zheng W. Physical activity, body size, and estrogen metabolism in women. Cancer Causes Control (Netherlands) Jun 2004;15(5):473-481.
- Yee GC, Stanley DL, Pessa LJ, et al. Effect of grapefruit juice on blood cyclosporine concentration. Lancet 1995;345:955-956.
- Guerra MC, Speroni E, Brocolli M, et al. Companion between Chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin. Antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Set 2000;67:2997-3006.
- Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol 1993;44:37-43.
- Pollard JW. Modifiers of estrogen action. Sci Med Jul/Aug 1999:38-47.
- Riby JE, Feng C, Chang YC, Schaldach CM, Firestone GL, Bjeldanes LF. The major cyclic trimeric product of indole-3-carbinol is a strong agonist of the estrogen receptor signaling pathway. Biochemistry (United States) Feb 8 2000;39(5):910-918.
- Meng Qj Yuan F, Goldberg ID, Rosen EM, Aubom K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr Dec 2000;130(12):292 7-2931.
- Yoon K, Pellaroni L, StonerM, Gaido K, Safe S. Differential activation of wild-type and variant forms of estrogen receptor a by synthetic and natural estrogenic compounds using a promoter containing three estrogen-responsive elements. J Steroid Biochem Mol Biol 2001;78:25-32.
- Yoon K, Pellaroni L, Ramamoorthy K, Gaido K, Safe S. Ligand structure-dependent differences in activation of estrogen receptor alfa in human HepG2 liver and U2 osteogenic cancer cell lines. Mol. Cell Endocrinol 2000;162:211-220.
- Bender DA. Novel function of vitamin B6. Proceedings Nutr Soc 1994;53:625-630.
- DeFlora S, Benniceli C, Camoiriano A et al. In vivo effects of N-acetylcysteine on glutatione metabolism and on biotransformation of carcinogen and/or mutagenic compounds. Carcinogenesis. 1985;6:1735-1745.
- Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disordes. BioDrugs 20()1;15(7): 465-489.
- Haberkorn V, HeydelJM, MounieJ, Artur Y, Gaudonnet H. Influence of vitamin A status on the regulation of uridine (5~~-) diphosphate-glucuronosyltransferase (UGT) 1A1 and UGT1A6 expression by L-triiodthyronine. British J Nutr 2001;85:289-297.
- Kassie F, Robot S, Uhl M et al. Chemoprotective effects of garden cress (Lepidium sativum) and its constituents towards 2-amino-3-methyl-imidazol[4,5-f]quinolin (IQJ-induced genotoxic effects and colonic preneoplastic lesions. Carcinogenesis 2002;23(7):1155 -1161.
- KirchhoffR Beckers CH, KirchhoffGM, Trinczek-Gartner H. Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomed 1994;1:107-115.
- Goldin BR Adlercreutz H, Gorbach SL. et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. New Engl J Med 1982;307:1542-1547.
- Dwivedi C, Heck Wf, Downie AA, Larroya S, Webb TE. Effect of calcium glucarate on fl-glucoronidase activity and glucarate content of certain vegetables and fruits. Biochem Med Metab Biol 1990;43:83-92.
- KumagaiA, Nishino K, Shimomura A, Kin T, Yamamura. Effect of glycyrrhizin on estrogen action. Endocrinol Japan 1967;14(l):34-38.
- HeckerM, Park JW, Murphy MB, Jones PD, Solomon KR, Van DerKraak G, CanJ A, Smith EE, du Preez L, Kendall RJ, Giesy JP. Effects of atrazine on CYP19 gene expression and aromatase activity in tests and on plasma sex steroid concentrations of male African clawed frogs (Xenopus laevis). Toxicol Sci (United States) Aug 2005;86(2):273-280.
- Holloway AC, Stys KA, Foster. DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine (United States) Jun 2005;27(1):45-50.
- Sanderson JT, Boerma J, Lansbergen GW, van den Berg M, Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol (United States) Jul 1 2002;182(l):44-54.
- Sanderson JT, HordijkJ, Denison MS, Springs teel MF, Nantz MH, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci (United Stales) Nov 2004;82(l):70-79.
- Etique N, Chardard D, Chesnel A, Merlin JE, Flament S, Grillier-Vuissoz I. Ethanol stimulates proliferation, ERalpha and aromatase expression in MCF-7 human breast cancer cells. Int J Mol Med (Greece) Jan 2004;13(l):149-55.
Lack of estrogen
Lack of female hormones in childhood is the reason for slow development:
- mammary glands;
- female genital organs;
- skeleton.
If adolescents have a lack of estrogen after puberty, the following symptoms may occur:
- reduction of the mammary glands (after the breasts have grown, they begin to shrink);
- absence of menstruation;
- reduction in the size of the uterus.
If a woman of childbearing age is estrogen deficient, the most common symptoms include:
- sudden change in mood;
- “coldness” in bed;
- irregular monthly cycles;
- pain in the lower abdomen during menstruation;
- insomnia;
- decreased performance;
- memory impairment;
- skin problems.
With a lack of hormones, inflammation and stretch marks may appear on the skin, and elasticity may decrease. As you can see, problems due to hormonal imbalance are mainly “female”: these hormones help us to be more feminine and beautiful.
Possible Health Problems with Low Estrogen Levels
Most often, problems bother women when estrogen levels are elevated, but there are often cases when the condition worsens even when it decreases.
Symptoms characteristic of decreased hormone levels:
- vaginal dryness or lack of lubrication;
- recurrent inflammatory diseases that become chronic;
- disturbances during the menstrual cycle;
- peeling and dry skin;
- change in emotional background.
Important! Abdominal pain during menstrual bleeding is not normal. Very often this indicates a decrease in estrogen.
How to increase estrogen?
If you notice the above signs of hormone deficiency, consult your doctor. Depending on the test results, he selects an individual way to increase hormones.
Doctors often prescribe tocopherol (vitamin E) to patients. It is also possible to take hormonal medications (oral contraceptives). Each tablet of combined oral contraceptives contains estrogen and progesterone (in different ratios).
You can also increase hormone levels in women with the help of food. They contain phytoestrogens - these are non-steroidal plant hormones, the structure of which is similar to human hormones. They contain:
- soybeans and soy products (milk, cheese, butter, flour, yogurt);
- other types of legumes (beans, peas, beans);
- products of animal origin (meat, fish oil, dairy products);
- some vegetables and fruits (carrots, red grapes, eggplants, tomatoes, pumpkin, cauliflower and Brussels sprouts);
- coffee.
If there is a lack of these hormones in the body, try to eat a dosed amount of these foods. Excessive portions can cause excess hormones.
The role of estradiol in the body of men and women
Estradiol in women has the following effects on the body:
• Increased voice timbre
• Features of the figure in the form of a narrow waist and fat distribution in the hips and buttocks;
• Estradiol in women affects the condition of the skin, making it thin and smooth;
• The growth of the follicle in the ovary, as one of the most important stages of the reproductive system, is also under the influence of this hormone;
• An increase in the volume of the uterine mucosa, its preparation for egg implantation and pregnancy;
• Regulation of the menstrual cycle.
If estradiol is low, all these functions that are essential for a woman’s health will be impaired.
Estradiol is also produced in men, but not in such large quantities as in women. Its main effects in men:
• Improvement of calcium deposition in bones, so low estradiol can lead to osteoporosis - thinning of bone tissue, increased bone fragility;
• Improving cell respiration and oxygen metabolism in the body;
• Participation in the production of seminal fluid, therefore, if estradiol is low, this can lead to decreased sperm quality and infertility;
• Stimulation of metabolism;
• Increased blood clotting;
• Reducing the level of “bad” cholesterol in the blood and protecting blood vessels and the heart from atherosclerosis and other diseases.
Most of these effects also apply to women's health. Therefore, if estradiol is increased or decreased, specialist supervision and consultation with an endocrinologist or gynecologist are required. Tests for estradiol, the norm of which depends on many factors, should only be deciphered by a doctor who takes into account the characteristics of your age, health, etc., and selects treatment based on objective data obtained.
Excess estrogen
With an increased amount of these hormones in the body, the following side effects may occur:
- nausea, vomiting;
- headache and dizziness;
- insomnia;
- irritability;
- soreness of the mammary glands;
- swelling, including bloating;
- high blood pressure;
- irregular periods;
- cold extremities (arms, legs);
- weight gain;
- fatigue;
- acne;
- hair loss;
- blood clot formation;
- tumors (uterus, breast, endometrium).
Both excess and deficiency of these hormones immediately manifest themselves externally and internally. In Russia, a lack of female hormones is rare, but an excess of them is very common. This is why Russian women are often diagnosed with breast cancer, mastopathy, and severe premenstrual syndrome.
If you experience the symptoms described, contact your doctor immediately. Adjust your hormonal levels before the situation gets worse.
hormone, estrogen, hormones
Treatment
To reduce estrogen levels, hormone therapy is usually used. Patients may be prescribed antiestrogenic drugs and progesterone preparations. To maintain normal hormone levels, the body needs B vitamins (especially B6), magnesium, and zinc.
To normalize estrogen levels it is recommended:
- adjust weight;
- avoid exposure to xenoestrogens (substances that have an estrogenic effect in the body), which are included in some cosmetics, plastics, and contraceptives;
- avoid stressful situations;
- normalize the daily routine, adhere to an adequate work and rest schedule;
- to refuse from bad habits;
- provide adequate physical activity;
- eat right, include foods rich in antioxidants and vitamins in your diet.
In some cases, complex treatment is required, aimed, for example, at normalizing the function of the thyroid gland and other endocrine organs.