Gel Venolife: composition
Venolife gel is a medicinal product for external use, containing three active components (heparin, dexpanthenol, troxerutin), and has 5 actions at once in the fight against varicose veins:
- reduction of swelling due to improved local blood flow and microcirculation;
- analgesic effect (reduction of pain caused by the inflammatory process);
- reduction of inflammation;
- restoration of damaged skin;
- strengthening capillaries.
The drug contains 3 active substances:
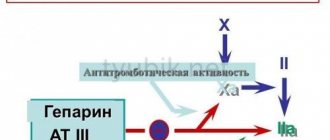
- Heparin. A direct-acting anticoagulant that has an anti-inflammatory effect, promotes tissue regeneration, prevents thrombus formation and improves local blood flow.
- Dexpanthenol. Provitamin B5 – turns into pantothenic acid in the skin, improving metabolic processes. Promotes regeneration of damaged tissues and improves heparin absorption.
- Troxerutin. Helps restore the firmness and elasticity of blood vessels, improving microcirculation and reducing venous stagnation. Has anti-edematous and anti-inflammatory effects.
Venolife gel 40g No. 1
Name
Venolife.
Release form
Gel for external use.
Dosage
40 g. Quantity per package: 1 pc.
Manufacturer
Akrikhin.
INN
Heparin sodium + dexpanthenol + troxerutin.
FTG
Venotonic and venoprotective agent.
Compound
1 g of gel contains: active substances: troxerutin – 0.02 g, sodium heparin – 0.0025 g (300 IU), dexpanthenol – 0.05 g; excipients: phenylethanol 0.01 g, propylene glycol 0.1 g, carbomer 940 or 980 0.008 g, trometamol 0.004 g, purified water up to 1 g.
Description
Transparent or almost transparent gel of light yellow color with a specific odor.
Pharmacotherapeutic group
Heparins or heparinoids for external use. ATX code: C05BA53
Pharmacological properties
Pharmacodynamics Venolife® gel is a combined preparation for external use, the pharmacological properties of which are determined by the action of the substances included in its composition. Heparin is a direct anticoagulant, a natural anticoagulant of the body, has an anti-inflammatory effect, promotes the regeneration of connective tissue by inhibiting the activity of hyaluronidase; prevents thrombus formation, activates fibrinolytic properties of blood; improves local blood flow. Dexpanthenol - provitamin B5 - is converted in the skin into pantothenic acid, which is part of coenzyme A, which plays an important role in the processes of acetylation and oxidation; by improving metabolic processes, dexpanthenol promotes the regeneration of damaged tissues; improves the absorption of heparin. Troxerutin is an angioprotective agent; has P-vitamin activity (reduces vascular tissue permeability and capillary fragility), helps normalize microcirculation and tissue trophism, reduces congestion in the veins and paravenous tissues; has antiexudative and anti-inflammatory effects. Pharmacokinetics: When applied topically, the active substances are quickly absorbed by the skin. Heparin accumulates in the upper layers and actively binds to skin proteins, dexpanthenol penetrates all layers of the skin and turns into pantothenic acid, troxerutin is found in the dermis after 30 minutes, and in subcutaneous fatty tissue after 2–5 hours.
Indications for use
As part of complex therapy: edema-pain syndrome and microcirculatory-trophic disorders caused by venous insufficiency of the lower extremities (varicose veins, chronic thrombophlebitis, post-thrombophlebitis syndrome, chronic lymphostasis); soft tissue injuries (hematomas, dislocations, sprains); to accelerate granulation and epithelization of trophic ulcers in the regeneration phase (in the absence of pronounced exudation).
Contraindications
in case of a history of heparin-induced thrombocytopenia type II (immune-mediated HIT type II); hypersensitivity to the components of the drug; open infected wounds or wounds with heavy exudation at the site of application; children under 18 years of age. Use in children There is no data on the use of troxerutin drugs in children.
Use during pregnancy and lactation
There is no data on the use of troxerutin drugs during pregnancy and lactation. Use is possible only after consulting a doctor, taking into account the balance of possible risk to the fetus/child and benefit to the woman.
Directions for use and doses
Externally. Apply a thin layer to the affected area 2-3 times a day, evenly distributing over the surface of the skin with light rubbing movements until completely absorbed. The course of treatment is 2–3 weeks. The need for further treatment is determined by the attending physician, based on the severity of the pathological process and the results of clinical tests. If the disease relapses, the course of treatment can be carried out 2-3 times a year. For trophic ulcers with weak exudation, before using the drug, the wound surface is cleaned of exudate and necrotic tissue, if necessary, washed with a solution of hydrogen peroxide 3%, nitrofural 1:5000 or chlorhexidine bigluconate 0.05% and dried. The gel is applied in a uniform thin layer so that the entire affected surface is covered with the drug, and a sterile gauze bandage is applied. The dressings are changed once a day. With the open method of treatment, the drug is applied 1-2 times a day. The duration of treatment is determined by the dynamics of epithelization. Special categories of patients Elderly: no dose adjustment required. Patients with impaired liver function: no dose adjustment is required. Patients with impaired renal function: no dose adjustment is required.
Side effect
Allergic reactions to heparin after applying the drug to the skin are very rare. However, in some cases, hypersensitivity reactions may occur, such as skin redness and itching, which quickly disappear after stopping use of the drug. Not known: Incidence of heparin-induced antibody-mediated thrombocytopenia type II (platelet count
Overdose
Overdose is unlikely due to the low systemic absorption of the gel components.
Precautionary measures
If there are signs of bleeding, the possibility of using Venolife® should be carefully considered. Venolife® should not be used for bleeding, applied to open wound surfaces, weeping eczema or mucous membranes, as well as to infected areas in the presence of purulent processes. Penetration of heparin into healthy skin has been reported with topical application, so if thromboembolic complications are suspected, platelet counts should be checked to differentiate heparin-induced thrombocytopenia type II. Therefore, regular platelet count monitoring in patients with a history of thromboembolic complications is necessary whenever heparin is used (before starting heparin, on the first day after starting use, and then regularly every 3–4 days during treatment until the end of treatment). Patients suffering from varicose veins of the lower extremities and venous insufficiency should strictly follow the additional therapeutic measures prescribed by the doctor, such as wearing compression medical hosiery (socks, stockings), compresses, a cool shower on the lower extremities, etc. If treatment is ineffective within 2 weeks of therapy, if the disease worsens or new symptoms appear, it is recommended to consult a doctor.
special instructions
Venolife® is not intended for use in ophthalmology, for intravaginal or rectal administration.
The ability to influence the reaction rate when driving vehicles and other mechanisms
The medicine does not affect the ability to drive a car or operate machinery.
Interaction with other drugs
Use with caution when taking oral anticoagulants simultaneously due to a possible enhancement of the effect. No interaction of the drug with other drugs has been identified.
Release form
Gel for external use. 40 g per aluminum tube. Each tube, along with instructions for use, is placed in a cardboard pack.
Storage conditions
At temperatures between 15 and 25 °C. Keep out of the reach of children.
Best before date
2 years. Do not use after the expiration date.
Conditions for dispensing from pharmacies
Over the counter.
Buy Venolife gel d/nar.prim. in tubes 40g per pack. No. 1 in pharmacy
Price for Venolife gel d/nar.approx. in tubes 40g per pack. No. 1
Instructions for use for Venolife gel d/nar.prim. in tubes 40g per pack. No. 1
Indications and contraindications
Venolife gel must be used according to the instructions for use in the following cases:
- To reduce swelling, pain in the legs, eliminate microcirculatory-trophic disorders that are caused by venous insufficiency (varicose veins, thrombophlebitis, chronic lymphostasis).
- Treatment of soft tissue injuries, including hematomas and sprains.
- To accelerate the healing of trophic ulcers.
Venolife gel can be used during pregnancy and lactation, as well as in children over 1 year of age.
The drug should not be used in the following cases:
- In case of individual sensitivity to the components included in the composition;
- In the presence of wounds with exudate or signs of infection.
Venolife 100g gel for external use
pharmachologic effect
Direct acting anticoagulant for local use + other drugs.
Composition and release form Venolife 100g gel for external use
Gel – 100 g:
- active ingredients: troxerutin in terms of 100% substance - 2 g, sodium heparin in terms of dry substance - 0.25 g (30000 IU), dexpanthenol in terms of 100% substance - 5 g;
- excipients: phenylethanol 1 g, propylene glycol 10 g, carbomer 940 or 980 0.8 g, trometamol 0.4 g, purified water up to 100 g.
40 or 100 g in an aluminum tube. Each tube, along with instructions for use, comes in a cardboard box.
Description of the dosage form
Transparent or almost transparent gel of light yellow color with a specific odor.
Directions for use and doses
Externally. Apply a thin layer to the affected area 2-3 times a day, evenly distributing over the surface of the skin with light rubbing movements until completely absorbed. The course of treatment is 2-3 weeks. The need for further treatment is determined by the attending physician, based on the severity of the pathological process and the results of clinical tests.
If the disease relapses, the course of treatment can be carried out 2-3 times a year.
For trophic ulcers with weak exudation, before using the drug, the wound surface is cleaned of exudate and necrotic tissue, if necessary, washed with a solution of hydrogen peroxide 3%, nitrofural 1:5000 or chlorhexidine bigluconate 0.05% and dried. The gel is applied in a uniform thin layer so that the entire affected surface is covered with the drug, and a sterile gauze bandage is applied. The dressings are changed once a day. With the open method of treatment, the drug is applied 1-2 times a day. The duration of treatment is determined by the dynamics of epithelization.
Pharmacodynamics
Venolife® gel is a combined preparation for external use, the pharmacological properties of which are determined by the action of the substances included in its composition.
Heparin is a direct anticoagulant, a natural anticoagulant of the body, has an anti-inflammatory effect, promotes the regeneration of connective tissue by inhibiting the activity of hyaluronidase; prevents thrombus formation, activates fibrinolytic properties of blood; improves local blood flow. Dexpanthenol - provitamin B5 - is converted in the skin into pantothenic acid, which is part of coenzyme A, which plays an important role in the processes of acetylation and oxidation; by improving metabolic processes, dexpanthenol promotes the regeneration of damaged tissues; improves the absorption of heparin.
Troxerutin is an angioprotective agent; has P-vitamin activity (reduces vascular tissue permeability and capillary fragility), helps normalize microcirculation and tissue trophism, reduces congestion in the veins and paravenous tissues, has an antiexudative and anti-inflammatory effect.
Pharmacokinetics
When applied topically, the active substances are quickly absorbed by the skin. Heparin accumulates in the upper layers and actively binds to skin proteins, dexpanthenol penetrates all layers of the skin and turns into pantothenic acid, troxerutin is found in the dermis after 30 minutes, and in subcutaneous fatty tissue after 2-5 hours.
Indications for use Venolife 100g gel for external use
Edema-pain syndrome and microcirculatory-trophic disorders caused by venous insufficiency of the lower extremities (varicose veins, acute and chronic thrombophlebitis, post-thrombophlebitis syndrome, chronic lymphostasis).
Soft tissue injuries (hematomas, dislocations, sprains).
To accelerate granulation and epithelization of trophic ulcers in the regeneration phase (in the absence of pronounced exudation).
Contraindications
Hypersensitivity to the components of the drug, open infected wounds or wounds with heavy exudation at the site of application, children under 1 year of age.
Application of Venolife 100g gel for external use during pregnancy and breastfeeding
Venolife® can be used during pregnancy and lactation.
special instructions
Venolife® is not intended for use in ophthalmology, for intravaginal or rectal administration.
Overdose
Overdose is unlikely due to the low systemic absorption of the gel components.
Side effects of Venolife 100g gel for external use
Local manifestations of allergic reactions (skin rash, itching) are possible.
Drug interactions
No interaction of the drug with other drugs has been identified.
Venolife gel external 100 g tubes al/pack cards x1
Trade name: Venolife International name: Heparin sodium + Dexpanthenol + Allantoin (Heparin sodium + Dexpanthenol + Allantoin) Pharmacological group: direct-acting anticoagulant for local use + other drugs Pharmacological group for ATC: C05BA53. Heparin in combination with other drugs Pharmacological action: analgesic, local anticoagulant, antiseptic, microcirculation improving, local anti-inflammatory Pharmacodynamics: Combined drug. Heparin sodium is a direct-acting anticoagulant, it also has an anti-inflammatory effect, promotes the regeneration of connective tissue by inhibiting the activity of hyaluronidase, prevents thrombus formation, activates the fibrinolytic system of the blood, and improves local blood flow. Dexpanthenol is provitamin B5, which is converted in the skin into pantothenic acid, which is part of coenzyme A, which plays an important role in the processes of acetylation and oxidation, improving metabolic processes, promoting the regeneration of damaged tissues, and improving the absorption of heparin. Troxerutin is an angioprotective agent, has P-vitamin activity (reduces vascular-tissue permeability and capillary fragility), helps normalize microcirculation and tissue trophism, reduces congestion in the veins and paravenous tissues, and has an anti-exudative and anti-inflammatory effect. Indications for use: Symptomatic treatment of venous insufficiency (varicose veins, acute and chronic thrombophlebitis, postthrombophlebitis syndrome, chronic lymphostasis). Soft tissue injuries (hematoma, swelling, including dislocation and sprain). Trophic ulcers in the regeneration phase (in the absence of pronounced exudation). Contraindications: Hypersensitivity, open infected wounds or wounds with heavy exudation. Dosage regimen: Externally, apply a thin layer to the affected area and around it, evenly distributing over the surface of the skin with light circular movements until completely absorbed 2-3 times a day. The course of treatment is 2-3 weeks, repeated 2-3 times a year (in case of relapse of the disease). For trophic ulcers with weak exudation, before use, the wound surface is cleaned of exudate and necrotic tissue, if necessary, washed with a solution of hydrogen peroxide 3%, nitrofural 1:5000 or chlorhexidine 0.05% and dried. The gel is applied in an even thin layer over the entire affected surface and a sterile gauze bandage is applied. The dressing is changed once a day. With the open method of treatment, the drug is applied 1-2 times a day. The duration of treatment is determined by the dynamics of epithelization. Side effects: Local allergic reactions (skin rash, itching). Special instructions: Not intended for use in ophthalmology, for intravaginal and rectal administration. Can be used during pregnancy and lactation.
Manufacturer: Akrikhin KhFK OJSC, Russia Registration certificate holder: Akrikhin KhFK OJSC, Russia Forms of release: gel for external use, aluminum tubes, gel for external use, aluminum tube. Composition: heparin sodium 250 mg = 30 thousand IU, dexpanthenol 5 g, troxerutin 2 g - 100 g Shelf life: 2 years Registration data: LS-001377 dated 07/14/2011, 03/10/2006 Status of the registration certificate: valid Pharmaceutical article number: FSP 42-0017-7082-05