Lancid Keith
Lancid Kit set of tablets and capsules x56, ATX code: A02BD (Combinations of drugs for the eradication of Helicobacter pylori)
Active substances
clarithromycin Rec.INN WHO registered amoxicillin Rec.INN WHO registered lansoprazole Rec.INN WHO registered
Dosage form
LANCID® KIT
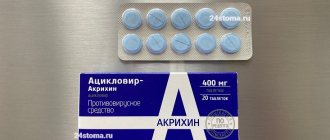
tablets and capsules set: blister, including: tab. clarithromycin, coating film-coated, 500 mg (2 pcs.), caps. amoxicillin 500 mg (4 pcs.), caps. lansoprazole enteric 30 mg (2 pcs.), 7 blisters per packagereg. No.: LP-002364 dated 02/10/14 - Valid
Release form, composition and packaging
Blister set of tablets and capsules contains:
Yellow film-coated tablets, oval-shaped, with a score line on one side, the color of the tablets at the break is white (2 pcs. in a blister).
1 tab.
clarithromycin 500 mg
Excipients: microcrystalline cellulose 31.5 mg, corn starch 8.9 mg, sorbic acid 1.1 mg, sorbitan a oleate 2 mg, povidone 30 mg, colloidal silicon dioxide 8 mg, magnesium stearate 11 mg, talc 24 mg, croscarmellose sodium 55 mg, stearic acid 20 mg.
Film shell composition: hypromellose 20.65 mg, titanium dioxide 4.75 mg, propylene glycol 3.2 mg, quinoline yellow dye 0.195 mg, vanilla flavor 1.2 mg.
Hard gelatin capsules, yellow body, dark red cap, on the body there is the inscription “500”, on the cap there is the inscription “AMOXI”, made in black ink, capsule size - No. 0 (4 pieces in a strip).
1 caps.
amoxicillin 500 mg
which corresponds to the content of amoxicillin trihydrate 588 mg
Excipients: magnesium stearate 5 mg, talc 8 mg, sodium lauryl sulfate 3 mg.
Composition of the capsule cap: propyl parahydroxybenzoate 0.2 mg, methyl parahydroxybenzoate 0.8 mg, water 14-15 mg, gelatin qs, titanium dioxide 0.8132 mg, brilliant blue dye 0.0062 mg, sunset yellow dye 0.0495 mg. Composition of the capsule body: propyl parahydroxybenzoate 0.2 mg, methyl parahydroxybenzoate 0.8 mg, water 14-15 mg, gelatin qs, titanium dioxide 1.6266 mg, iron dye yellow oxide 0.9999 mg. Composition of black ink: ethanol 29-33%, isopropanol 9-12%, butanol 4-7%, shellac 24-28%, black iron oxide dye 24-28%, aqueous ammonia 1-3%, propylene glycol 0.5-2%.
Enteric hard gelatin capsules, No. 1, with a pink body and cap and a black inscription “MICRO/MICRO”, the contents of the capsules are granules (pellets) of white or almost white color (2 pieces in a strip).
1 caps.
lansoprazole 30 mg
Excipients: mannitol 41.11 mg, sucrose 123.22 mg, povidone 1.09 mg, sucrose microspheres 38.19 mg, sodium hydrogen phosphate 2.08 mg, calcium carmellose 10.41 mg, magnesium hydroxycarbonate 5.3 mg, polysorbate 80 0.99 mg, hypromellose 25.58 mg, titanium dioxide 2.19 mg, methacrylic acid polymer 65.78 mg, talc 8.77 mg, diethyl phthalate 8.11 mg, sodium hydroxide 0.44 mg.
Capsule body composition: gelatin 38.9575 mg, sodium lauryl sulfate 0.0376 mg, propyl parahydroxybenzoate 0.376 mg, methyl parahydroxybenzoate 0.094 mg, titanium dioxide 0.712 mg, crimson dye (Ponceau 4R) 0.0078 mg, water 6.815 mg. Composition of the capsule cap: gelatin 24.0376 mg, sodium lauryl sulfate 0.0232 mg, propyl parahydroxybenzoate 0.058 mg, methyl parahydroxybenzoate 0.232 mg, titanium dioxide 0.4393 mg, crimson dye (Ponceau 4R) 0.0048 mg, water 4.205 mg.
8 pcs. - blisters (7) - cardboard packs.
Clinical and pharmacological group: Antiulcer drug that has a bactericidal effect on Helicobacter pylori Pharmacotherapeutic group: Antiulcer drug
pharmachologic effect
Pharmacokinetics,
Indications
- peptic ulcer of the stomach and duodenum (treatment and eradication therapy for Helicobacter pylori infection).
ICD-10 codes,
Dosage regimen
Inside. Take 500 mg (1 tablet) of clarithromycin, 1000 mg of amoxicillin (2 capsules) and 30 mg of lansoprazole (1 capsule), twice a day in the morning and evening before meals.
Tablets and capsules should not be broken or chewed and should be swallowed whole. The duration of treatment is 7 days, if necessary it can be increased to 14 days.
Each blister of Lancid® Kit contains two tablets of clarithromycin (500 mg), four capsules of amoxicillin (500 mg) and 2 capsules of lansoprazole (30 mg) and is designed for one day of treatment. One package contains 7 blisters and is designed for one course of treatment.
Side effect
The adverse events listed below are distributed according to the frequency of occurrence according to the following gradation: very often (≥ 1/10), often (from ≥ 1/100 to < 1/10), infrequently (from ≥ 1/1000 to < 1/10). 100), rare (from ≥ 1/10000 to <1/1000), very rare (<1/10000).
Clarithromycin
Infectious and parasitic diseases: uncommon - candidiasis, gastroenteritis, development of superinfection (with prolonged or repeated use of clarithromycin), vaginal infections, frequency unknown - pseudomembranous colitis, erysipelas, erythrasma.
Disorders of the blood and lymphatic system: infrequently - leukopenia, neutropenia, thrombocythemia, eosinophilia, frequency unknown - agranulocytosis, thrombocytopenia.
Immune system disorders: uncommon - hypersensitivity, frequency unknown - anaphylactic reactions.
Metabolic and nutritional disorders: uncommon - anorexia, loss of appetite, frequency unknown - hypoglycemia (including when taking hypoglycemic drugs simultaneously).
Mental disorders: often - insomnia, infrequently - anxiety, nervousness, frequency unknown - psychosis, confusion, depersonalization, depression, disorientation, hallucinations, nightmares, mania.
Nervous system disorders: often - changes in taste (dysgeusia), headache, infrequently - dizziness, loss of consciousness, drowsiness, tremor, frequency unknown - convulsions, loss of taste, impaired sense of smell, loss of smell, paresthesia.
Hearing disorders and labyrinthine disorders: infrequently - vertigo, hearing impairment, noise, ringing in the ears, frequency unknown - hearing loss (passing after discontinuation of the drug).
Cardiac disorders: uncommon - prolongation of the QT interval on the electrocardiogram, palpitations, frequency unknown - ventricular tachycardia of the pirouette type, ventricular tachycardia, ventricular flutter and fibrillation.
Vascular disorders: frequency unknown - unusual bleeding, hemorrhage.
Disorders of the respiratory system, chest and mediastinal organs: infrequently - nosebleeds.
Gastrointestinal tract disorders: often - diarrhea, vomiting, dyspepsia, nausea, abdominal pain, infrequently - gastroesophageal reflux disease, gastritis, proctalgia, stomatitis, glossitis, bloating, constipation, dry mouth, belching, flatulence, frequency unknown - acute pancreatitis, discoloration of the tongue and teeth.
Disorders of the liver and biliary tract: often - atypical liver function test, infrequently - cholestasis, hepatitis, increased ALT activity, increased AST activity, increased GGT activity, frequency unknown - liver failure, hepatocellular jaundice.
Disorders of the skin and subcutaneous tissues: often - rash, increased sweating, infrequently - itching, urticaria, maculopapular rash, frequency unknown - malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), drug rash with eosinophilia and systemic manifestations, acne, Henoch-Schönlein purpura.
Musculoskeletal and connective tissue disorders: uncommon - muscle spasm, myalgia, frequency unknown - rhabdomyolysis, myopathy, increased symptoms of myasthenia gravis.
Renal and urinary tract disorders: very rarely - renal failure, interstitial nephritis.
General disorders and disorders at the injection site: uncommon - malaise, fever, asthenia, chest pain, chills, weakness.
Laboratory indicators: infrequently - increased alkaline phosphatase activity, increased blood LDH activity, very rarely - hypercreatininemia, frequency unknown - increased international normalized ratio (INR), increased prothrombin time, change in urine color, increased bilirubin concentration.
Amoxicillin
Infectious and parasitic diseases: uncommon - development of superinfection, candidiasis of the oral mucosa, vaginal candidiasis.
Blood and lymphatic system disorders: rarely - eosinophilia, hemolytic anemia, very rarely - leukopenia, neutropenia, grayulocytopenia, thrombocytopenia, pancytopenia, anemia, myelosuppression, agranulocytosis, reversible increase in prothrombin time and bleeding time.
Immune system disorders: rarely - laryngeal edema, serum sickness, allergic purpura, anaphylactic reaction.
Nervous system disorders: uncommon - headache, rare - agitation, anxiety, insomnia, ataxia, confusion, hyperkinesia, behavior changes, depression, peripheral neuropathy, dizziness, convulsions (in patients with impaired renal function, epilepsy or meningitis).
Gastrointestinal disorders: often - nausea, loss of appetite, vomiting, flatulence, soft stools, diarrhea, rash on the oral mucosa, dry mouth, distorted taste perception, rarely - darkening of tooth enamel, very rarely - pseudomembranous colitis , black "hairy" tongue.
Disorders of the liver and biliary tract: infrequently - reversible increase in the activity of “liver” transaminases, rarely - hepatitis, cholestatic jaundice.
Disorders of the skin and subcutaneous tissues: often - skin rashes, itching, urticaria, rarely - angioedema (Quincke's edema), polymorphic exudative erythema, acute generalized exanthematous pustulosis, toxic epidermal necrolysis (Lyell's syndrome), malignant exudative erythema (Stevens syndrome). Johnson), bullous and exfoliative dermatitis.
Renal disorders: rarely - acute interstitial nephritis, crystalluria.
General disorders and disorders at the injection site: rarely - drug fever.
Lansoprazole
Disorders of the blood and lymphatic system: infrequently - thrombocytopenia, eosinophilia, leukopenia, rarely - anemia, very rarely - agranulocytosis, pancytopenia.
Immune system disorders: very rarely - anaphylactic shock.
Metabolic and nutritional disorders: rarely - anorexia, frequency unknown - hypomagnesemia.
Mental disorders: infrequently - depression, rarely - insomnia, hallucinations, confusion.
Nervous system disorders: often - headache, dizziness, rarely - anxiety, vertigo and paresthesia, drowsiness, tremor.
Visual disturbances: rarely - visual disturbances.
Gastrointestinal disorders: often - nausea, diarrhea, abdominal pain, constipation, vomiting, flatulence, dry mouth or throat, rarely - glossitis, esophageal candidiasis, pancreatitis, impaired taste perception, very rarely - colitis, stomatitis.
Disorders of the liver and biliary tract: often - increased activity of liver transaminases, rarely - hepatitis, jaundice, very rarely - hyperbilirubinemia.
From the respiratory system: rarely - cough, pharyngitis, rhinitis, upper respiratory tract infection, influenza-like syndrome.
Disorders of the skin and subcutaneous tissues: often - urticaria, itching, rash, rarely - petechiae, purpura, alopecia, angioedema (Quincke's edema), erythema multiforme, photosensitivity, very rarely - malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome).
Musculoskeletal and connective tissue disorders: uncommon - arthralgia, myalgia, fracture of the hip, wrist or spine.
Renal and urinary tract disorders: rarely - interstitial nephritis.
Disorders of the genital organs and mammary gland: rarely - gynecomastia, impotence.
General disorders and disorders at the injection site: often - weakness, infrequently - swelling, rarely - fever, increased sweating.
Laboratory indicators: very rarely - increased cholesterol and triglyceride levels, hyponatremia.
Contraindications for use
- hypersensitivity to any component of the drugs (main substance and/or auxiliary components), to macrolides, to penicillins, cephalosporins, carbapenems,
- simultaneous use of clarithromycin with the following drugs: astemizole, cisapride, pimozide, terfenadine, with ergot alkaloids, for example, ergotamine, dihydroergotamine, with midazolam for oral use,
- simultaneous use of clarithromycin with HMG-CoA reductase inhibitors (statins), which are largely metabolized by the CYP3A4 isoenzyme (lovastatin, simvastatin), due to an increased risk of myopathy, including rhabdomyolysis,
- simultaneous use of clarithromycin with colchicine in patients with impaired liver and kidney function,
- patients with a history of QT interval prolongation, ventricular arrhythmia or torsade de pointes (TdP),
- patients with hypokalemia (risk of QT interval prolongation),
- patients with severe liver failure occurring simultaneously with renal failure,
- patients with a history of cholestatic jaundice/hepatitis that developed while using clarithromycin,
- with porphyria,
- during breastfeeding and pregnancy,
- patients with atopic dermatitis, bronchial asthma, hay fever, infectious mononucleosis, lymphocytic leukemia, liver failure, gastrointestinal diseases in history (especially colitis associated with the use of antibiotics),
- children under 18 years of age,
- in the presence of sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption.
Carefully
Moderate to severe renal failure, moderate to severe liver failure, myasthenia gravis (possible increased symptoms), concomitant use with drugs that are metabolized by the CYP3A isoenzyme (for example, carbamazepine, cilostazol, cyclosporine, disopyramide, methylprednisolone, omeprazole, indirect anticoagulants (for example , warfarin), quinidine, rifabutin, sildenafil, tacrolimus, vinblastine), concomitant use with drugs that induce the CYP3A4 isoenzyme (for example, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort), concomitant use with benzodiazepines such as alprazolam, triazolam, midazolam for intravenous use, simultaneous use with calcium channel blockers that are metabolized by the CYP3A4 isoenzyme (for example, verapamil, amlodipine, diltiazem), patients with coronary heart disease (CHD), severe heart failure, hypomagnesemia, severe bradycardia (less than 50 beats/min), as well as patients simultaneously taking class IA (quinidine, procainamide) and class III antiarrhythmic drugs (dofetilide, amiodarone, sotalol), older age, a history of bleeding, allergic reactions (including a history), concomitant therapy with clopidogrel.
Use during pregnancy and breastfeeding
Lancid® Kit is contraindicated during pregnancy and breastfeeding.
Use for liver dysfunction Use with caution in moderate to severe liver failure.
Use for renal impairment
Use with caution in moderate to severe renal failure.
Use in children The use of the drug in children under 18 years of age is contraindicated. ,
Use in elderly patients The drug should be used with caution in elderly patients. ,
special instructions
Before starting therapy, it is necessary to exclude the presence of a malignant process (especially with a stomach ulcer), since treatment, masking symptoms, can delay the correct diagnosis.
Clarithromycin
Long-term use of antibiotics can lead to the formation of colonies with an increased number of insensitive bacteria and fungi. In case of superinfection, appropriate therapy must be prescribed.
Hepatic dysfunction (increased plasma levels of liver enzymes, hepatocellular and/or cholestatic hepatitis with or without jaundice) has been reported with the use of clarithromycin. Liver dysfunction can be severe but is usually reversible. There have been cases of fatal liver failure, mainly associated with the presence of serious concomitant diseases and/or concomitant use of other drugs. If signs and symptoms of hepatitis such as anorexia appear. jaundice, dark urine, abdominal tenderness on palpation, clarithromycin therapy should be stopped immediately.
In the presence of chronic liver diseases, it is necessary to regularly monitor blood plasma enzymes.
When treated with almost all antibacterial agents, including clarithromycin, cases of pseudomembranous colitis have been described, the severity of which can range from mild to life-threatening.
Antibacterial drugs can change the normal intestinal microflora, which can lead to the growth of Clostridium difficile. Pseudomembranous colitis caused by Clostridium difficile should be suspected in all patients who experience diarrhea after using antibacterial agents. After a course of antibiotic therapy, careful medical monitoring of the patient is necessary. Cases of the development of pseudomembranous colitis 2 months after taking antibiotics have been described.
Clarithromycin should be used with caution in patients with coronary heart disease (CHD), severe heart failure, hypomagnesemia, severe bradycardia (less than 50 beats/min), as well as when used simultaneously with class IA (quinidine, procainamide) and class III antiarrhythmic drugs ( dofetilide, amiodarone, sotalol). In these conditions and while taking clarithromycin with these drugs, the electrocardiogram should be regularly monitored for an increase in the QT interval.
It is possible to develop cross-resistance to clarithromycin and other macrolide antibiotics, as well as lincomycin and clindamycin.
If acute hypersensitivity reactions occur, such as anaphylactic reaction, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms (DRESS syndrome), Henoch-Schönlein purpura, you should immediately stop taking clarithromycin and begin appropriate therapy.
Worsening of myasthenia gravis symptoms has been reported in patients taking clarithromycin.
In case of combined use with warfarin or other indirect anticoagulants, it is necessary to monitor MHO and prothrombin time.
Amoxicillin
Before starting amoxicillin, it is necessary to obtain a detailed history regarding previous hypersensitivity reactions to penicillins, cephalosporins or other allergens. Serious and sometimes fatal hypersensitivity reactions (anaphylactic reactions) to penicillins have been described. The risk of such reactions is highest in patients with a history of hypersensitivity reactions to penicillins. If allergic reactions occur, you must stop taking amoxicillin and start therapy with an antibiotic from a different group. In case of serious hypersensitivity reactions, appropriate measures should be taken immediately. Epinephrine, oxygen therapy, intravenous corticosteroids, and airway management, including intubation, may also be required.
It is necessary to refrain from using amoxicillin if infectious mononucleosis is suspected, since amoxicillin can cause a measles-like skin rash in patients with this disease, making diagnosis difficult.
Long-term treatment with amoxicillin sometimes leads to excessive proliferation of insensitive microorganisms.
During the use of amoxicillin, it is recommended to periodically evaluate renal, hepatic and hematopoietic function. Amoxicillin should be used with caution in patients with impaired liver function. Liver function should be monitored on a regular basis. In patients with impaired renal function, the dose of amoxicillin should be reduced according to the degree of impairment.
Amoxicillin can provoke nonspecific binding of immunoglobulins and albumins to the erythrocyte membrane, which can cause a false-positive reaction with the Coombs test.
Crystalluria very rarely occurs in patients with reduced diuresis. Adequate fluid intake and maintaining adequate diuresis are extremely important during amoxicillin therapy.
In patients with cholangitis or cholecystitis, antibiotics can be prescribed only if the disease is mild and in the absence of cholestasis. During therapy with amoxicillin, it is necessary to remember the possible development of superinfection (usually caused by bacteria of the genus Pseudomonas spp. or fungi of the genus Candida). In this case, amoxicillin therapy should be discontinued and/or appropriate treatment should be prescribed.
If severe diarrhea persists, pseudomembranous colitis caused by antibiotics should be suspected, which can pose a threat to the patient's life (watery stool mixed with blood and mucus, dull widespread or colicky abdominal pain, fever, and sometimes tenesmus). In such cases, amoxicillin should be immediately discontinued and pathogen-specific treatment, such as vancomycin, should be prescribed. In this case, drugs that reduce gastrointestinal perilstatics are contraindicated. The excretion of amoxicillin leads to its high content in the urine, which can lead to false-positive results when determining glucose in urine (for example, Benedict's test, Fehling's test). In this case, it is recommended to use the glucose oxidase method for determining the concentration of glucose in urine.
If concomitant use of amoxicillin with anticoagulants is necessary, prothrombin time or INR should be carefully monitored when amoxicillin is prescribed or discontinued.
When using estrogen-containing oral contraceptives and amoxicillin simultaneously, other or additional methods of contraception should be used.
Particular caution is recommended for patients with allergic diathesis or bronchial asthma, or a history of gastrointestinal diseases (in particular, colitis caused by antibiotic treatment).
When taking amoxicillin for a long time, nystatin, levorin or other antifungal drugs should be prescribed simultaneously.
It is not recommended to drink alcohol during treatment.
Lansoprazole
It is recommended to avoid the combined use of proton pump inhibitors and clopidogrel. When used together, the risk of recurrent myocardial infarction, hospitalization for a heart attack or unstable angina, stroke, and repeated revascularization increases. If co-administration is absolutely necessary, patients should be closely monitored.
It is recommended to avoid the concomitant use of proton pump inhibitors and antiretroviral drugs in HIV-infected patients. If combined use with atazanavir/ritonavir is necessary, it is recommended to maintain a 12-hour interval between taking lansoprazole and these drugs, and not to exceed the dose of lansoprazole 30 mg.
When used together with antiretroviral drugs (indinavir, nelfinavir, atazanavir), as well as ketoconazole, itraconazole, posaconazole, cefpodoxime, cefuroxime and ampicillin, monitoring of their effectiveness and the emergence of resistance is necessary.
Concomitant use with imatinib may increase the risk of adverse reactions (potential interaction via CYP3A4), especially in individuals with a history of severe allergic reactions.
Due to the increased risk of myotoxicity, patients taking atorvastatin, lovastatin, or simvastatin should be closely monitored during concomitant use of lansoprazole.
In patients taking warfarin concomitantly, monitoring of prothrombin time and MHO is necessary.
Long-term use of proton pump inhibitors increases the risk of infection (including Salmonella, Campylobacter, Clostridium difficile). The benefit of preventing upper gastrointestinal bleeding must be weighed against the potential risk of developing ventilator-associated pneumonia.
Long-term use of proton pump inhibitors increases the risk of fractures in postmenopausal women.
During the treatment period, you should avoid drinking alcoholic beverages.
Pharmacogenetic factor. The effectiveness of the drug depends on the genetic polymorphism of CYP2C19. In patients classified as “slow metabolizers” (RM-type), the effectiveness is higher, eradication of Helicobacter pylori is significantly more often achieved compared to “fast metabolizers” (homEM-type), even against the background of resistance to clarithromycin.
“The syndrome is not typical for lansoprazole if the recommendations for the duration of use are followed.
Effect on the ability to drive machinery and a car
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, as the drug can cause weakness, drowsiness and dizziness.
Overdose
Clarithromycin
Symptoms: abdominal pain, nausea, vomiting, diarrhea, possible headache, confusion.
Treatment: gastric lavage, maintenance therapy. It is not removed by hemo- or peritoneal dialysis.
Amoxicillin
Symptoms: nausea, vomiting, diarrhea, disorders
Lancid® Kit
Clarithromycin
When taking clarithromycin together and drugs that are primarily metabolized by CYP3A isoenzymes, a mutual increase in their concentrations is possible, which can enhance or prolong both therapeutic and side effects.
Contraindicated
combined use with astemizole, cisapride, pimozide, terfenadine, ergotamine and other ergot alkaloids, alprazolam, midazolam, triazolam.
Carefully
prescribed with carbamazepine, cilostazol, cyclosporine, disopyramide, lovastatin, methylprednisolone, omeprazole, indirect anticoagulants (including warfarin), quinidine, rifabutin, sildenafil, simvastatin, tacrolimus, vinblastine, phenytoin, theophylline and valproic acid (metabolized through other cytochrome P450 isoenzymes ). It is necessary to adjust the dose of drugs and control the concentration in the blood plasma.
When used together with cisapride, pimozide, terfenadine and astemizole, it is possible to increase the concentration of the latter in the blood plasma, prolong the QT interval and develop cardiac arrhythmias, including ventricular paroxysmal tachycardia, fibrillation, ventricular flutter or fibrillation, polymorphic ventricular tachycardia of the “pirouette” type (see. section "Contraindications"). A similar mechanism of interaction is observed when using drugs that are metabolized by another isoenzyme of the cytochrome P450 system - phenytoin, theophylline and valproic acid. When prescribing the above drugs simultaneously, monitoring of their concentrations in blood plasma and ECG is required.
Clarithromycin may decrease the clearance of triazolam and thus increase its pharmacological effects resulting in drowsiness and confusion.
For benzodiazepines whose elimination is not dependent on CYP3A4 isoenzymes (temazepam, nitrazepam, lorazepam), a clinically significant interaction with clarithromycin is unlikely.
There are reports of increased concentrations of digoxin in the blood plasma of patients receiving concomitant digoxin and clarithromycin. Digoxin plasma levels should be constantly monitored to avoid digitalis intoxication and the development of potentially fatal arrhythmias.
Concomitant use with ergotamine and dihydroergotamine (ergot derivatives) can lead to acute ergotamine intoxication, manifested by severe peripheral vasospasm, ischemia of the limbs and other tissues, including the central nervous system, and perverse sensitivity.
When clarithromycin is taken concomitantly with HMG-CoA reductase inhibitors - lovastatin and simvastatin - rare cases of rhabdomyolysis have been described.
Efavirenz, nevirapine, rifampicin, rifabutin and rifapentine (cytochrome P450 inducers) reduce the concentration of clarithromycin in the blood plasma and weaken its therapeutic effect, and at the same time increase the concentration of 14(R)-hydroxyclarithromycin.
When co-administered with fluconazole at a dose of 200 mg and clarithromycin at a dose of 1 g/day, the steady-state concentration and AUC of clarithromycin may increase by 33% and 18%, respectively. No dose adjustment of clarithromycin is required.
Attention should be paid to the possibility of cross-resistance between clarithromycin and other macrolide antibiotics, as well as lincomycin and clindamycin.
Concomitant use of clarithromycin and zidovudine in adult HIV-infected patients may result in a decrease in the steady-state concentration of zidovudine. It is necessary to select doses of clarithromycin and zidovudine.
With the simultaneous administration of clarithromycin and ritonavir, atazanavir or other protease inhibitors, the plasma concentrations of both clarithromycin, which in this case should not be prescribed at a dose exceeding 1 g/day, and the protease inhibitor increase.
When clarithromycin and itraconazole are taken together, a mutual increase in the concentration of drugs in the blood plasma is possible. Patients taking itraconazole and clarithromycin concomitantly should be closely monitored due to the possible enhancement or prolongation of the pharmacological effects of these drugs.
When co-administered with clarithromycin (1 g/day) and saquinavir (soft gelatin capsules, 1200 mg 3 times daily), the AUC and steady-state concentration of saquinavir may increase by 177% and 187%, respectively, and clarithromycin by 40%. When these two drugs are prescribed together for a limited time in the doses/dosage forms indicated above, no dose adjustment is required. Since co-administration of colchicine, which is a substrate for CYP3A and P-glycoprotein, and clarithromycin, as well as other macrolide inhibitors of CYP3A and P-glycoprotein, inhibition may lead to increased effects of colchicine, patients should be carefully monitored for symptoms of colchicine toxicity. . When using clarithromycin with tolterodine in patients with low CYP2D6 activity, a dose reduction of tolterodine may be required in the presence of clarithromycin (an inhibitor of CYP3A isoenzymes).
When clarithromycin is taken together with verapamil, a decrease in blood pressure, bradyarrhythmia and lactic acidosis are possible.
When using etravirine, the concentration of clarithromycin decreases, but the concentration of the active metabolite 14(R)-hydroxy-clarithromycin increases.
When clarithromycin is used concomitantly with oral hypoglycemic agents and/or insulin, severe hypoglycemia may occur. During concomitant use of clarithromycin and certain drugs that lower glucose concentrations, such as nateglinide, pioglitazone, repaglinide and rosiglitazone, inhibition of the CYP3A isoenzyme by clarithromycin may occur, which may result in hypoglycemia. Careful monitoring of glucose concentrations is recommended.
Amoxicillin
Antacids, glucosamine, laxatives, aminoglycosides, food slow down and reduce the absorption of amoxicillin; ascorbic acid increases absorption.
Probenecid reduces the excretion of amoxicillin by the kidneys and increases the concentration of amoxicillin in bile and blood plasma.
Bactericidal antibiotics (including aminoglycosides, cephalosporins, vancomycin, rifampicin) - synergistic effect; bacteriostatic drugs (macrolides, chloramphinecol, lincosamides, tetracyclines, sulfonamides) - antagonistic.
When taking amoxicillin in combination with metronidazole, nausea, vomiting, anorexia, diarrhea, constipation, epigastric pain, digestive disorders, in rare cases, jaundice, interstitial nephritis, and hematopoietic disorders are observed.
Amoxicillin increases the effectiveness of indirect anticoagulants (suppressing intestinal microflora, reduces the synthesis of vitamin K and the prothrombin index), which leads to an increase in blood clotting time. If necessary, adjust the dose of indirect anticoagulants. Concomitant use of amoxicillin and allopurinol increases the risk of developing skin rash.
Amoxicillin reduces the clearance and increases the toxicity of methotrexate, probably due to the competitive inhibition of renal tubular secretion of methotrexate by amoxicillin. In patients receiving concomitant amoxicillin and methotrexate, plasma concentrations of the latter should be carefully monitored.
The absorption time of digoxin may increase during amoxicillin therapy. If necessary, adjust the dose of digoxin.
Diuretics, allopurinol, oxyphenbutazone, phenylbutazone, non-steroidal anti-inflammatory drugs and other drugs that block tubular secretion increase the concentration of amoxicillin in the blood plasma.
Amoxicillin reduces the concentration of estrogens and progesterones in the blood plasma, which can lead to loss of the contraceptive effect of oral contraceptives. Additional non-hormonal methods of contraception should be used during treatment with amoxicillin.
Lansoprazole
Lansoprazole slows down the elimination of drugs metabolized in the liver by microsomal oxidation (including diazepam, phenytoin, indirect anticoagulants).
Reduces the clearance of theophylline by 10%.
Slows down the pH-dependent absorption of drugs belonging to weak acid groups and accelerates the absorption of drugs belonging to base groups.
Interferes with the absorption of ketoconazole, itraconazole, ampicillin, iron salts, digoxin.
Lansoprazole slows down the absorption of cyanocobalamin.
Compatible with ibuprofen, indomethacin, diazepam, propranolol, warfarin, oral contraceptives, phenytoin, prednisolone. Sucralfate reduces the bioavailability of lansoprazole by 30%, so it is necessary to maintain an interval of 30-40 minutes between taking these medications.
Antacids should be prescribed 1 hour before or 1-2 hours after taking lansoprazole, as they slow down and reduce its absorption.
In volunteers who simultaneously received 60 mg of lansoprazole and 400 mg of atazanavir per day, there was a 90% decrease in AUC and Cmax of the latter. Lansoprazole should not be co-administered with atazanavir.
Ritonavir (a substrate and inhibitor of CYP2C19) may have a variable effect on the AUC of lansoprazole (increase or decrease). If concomitant therapy is necessary, monitoring of therapeutic and possible side effects, as well as dose adjustment of lansoprazole, is recommended. Simultaneous administration of lansoprazole and tacrolimus (a substrate of the CYP3A4 isoenzyme and P-glycoprotein) leads to an increase in the plasma concentration of the latter (up to 81%). Plasma concentrations of tacrolimus should be monitored when coadministered with lansoprazole.
Simultaneous administration of fluvoxamine (an inhibitor of the CYP2C19 isoenzyme) and lansoprazole leads to a fourfold increase in the concentration of the latter in the blood plasma.
Rifampicin and St. John's wort (induce CYP2C19 and CYP3A4 isoenzymes) can significantly reduce plasma concentrations of lansoprazole.