Introduction
Mercury is an ultramicroelement and is constantly present in the body through food and water. Mercury has no physiological function in the human body. It is highly toxic and cumulative. Mercury is widespread in all elements of the environment due to the high volatility of metal vapors, but local foci of anthropogenic pollution, which, unfortunately, are often found both in urban areas and in rural areas, are of hygienic importance.
Sources of anthropogenic environmental pollution with mercury are thermal power plants, non-ferrous metals factories, pulp and paper factories, and cement factories. Mercury intake associated with the use of mercury-containing agricultural fungicides is important. From atmospheric air, vapors and aerosols of mercury compounds enter water bodies as a result of sedimentation and precipitation.
First aid for poisoning
If a person has inhaled toxic fumes or been poisoned by harmful compounds, it is necessary to give him access to fresh air and urgently remove him from the contaminated area, preventing further poisoning.
Additionally, you need to remove clothing that may have gotten mercury on it. First aid for poisoning involves calling an ambulance, then rinsing the victim’s mucous membranes (eyes, nose, etc.).
While the victim is waiting for a doctor, he needs to be given a large amount of liquid, in particular milk.
It is not recommended to give medications without the supervision of a specialist, because an allergic reaction may begin.
Effects of mercury on the body
Mercury in the form of inorganic compounds
First of all, Hg cations react with the SH groups of proteins (“thiol poisons”), as well as with the carboxyl and amine groups of tissue proteins, forming strong complex compounds—metalloproteins. As a result, profound dysfunctions of the central nervous system, especially its higher parts, occur. Inorganic mercury compounds are characterized by damage to the kidneys and liver.
Mercury in the form of organic compounds
The most important is methylmercury, which is highly soluble in lipid tissues and quickly penetrates into vital organs, including the brain . As a result, changes occur in the autonomic nervous system, peripheral nerve formations, in the heart, blood vessels, hematopoietic organs, liver, etc., and disturbances in the immunobiological state of the body. Mercury compounds also have an embryotoxic effect ( lead to fetal damage in pregnant women ).
Inorganic mercury in natural waters is capable of methylation. It should be noted that the pathogenesis and clinical manifestations of intoxication with organic mercury compounds are fundamentally different from intoxication with inorganic mercury. Because of this, hygienic standards for inorganic mercury and its alkyl derivatives are different, which must be taken into account when organizing laboratory control of water quality. In a water supply contaminated with inorganic mercury compounds, naturally methylated (more toxic) mercury is thought to account for 0.1% of the total contamination. It follows from this that water containing inorganic mercury at the level of hygienic standards will also be safe with respect to alkylmercury.
The short video below clearly demonstrates the negative effects of mercury on brain cells and describes the mechanism of this effect:
Mercury in the hydrosphere
It is believed that by the end of the second millennium, about 50 million tons of mercury compounds had accumulated in the World Ocean, and the natural removal of mercury into the ocean as a result of erosion is approximately 5 thousand tons per year. Intense binding of mercury to solid suspended particles leads to a concentration factor of the order of 1.3–1.8×105, that is, the proportion of mercury associated with suspended particles (size less than 0.45 microns) is 10 thousand times greater than the dissolved fraction.
Of the few data available in the literature on the pollution of aquatic ecosystems with mercury compounds, the results of studying the influence of wastewater from the North Baikal branch of the Baikal-Amur Mainline on the pollution of Lake Baikal are of indisputable interest. This study showed that in the waters of Northern Baikal and the Tyya and Kichera rivers, mercury-containing compounds are found in concentrations of 0.1–0.2 μg/l. A significant contribution to the pollution of Lake Baikal with mercury since the beginning of production at the Baikal Pulp and Paper Mill (BPPM) has been made by wastewater from this plant.
In Russia, the heaviest pollution is observed near metallurgical plants on the Kola Peninsula and in Norilsk, where the corresponding concentrations exceed background levels by tens and in some places hundreds of times. Because lake sediments are excellent reservoirs of heavy metals, it is possible that these pollution levels will remain high for many decades.
The amount of mercury increases over time not only in lake sediments, but also in sea bottom sediments. Even at the North Pole, in sediments from depths of 22 to 3 m, mercury concentrations increase from 0.03 to 0.13 μg/kg. These data indicate an increasing global flux of mercury, which is deposited in the Arctic due to “polar distillation.”
What to do if the thermometer is broken
A broken thermometer may seem like a minor problem, but it's still a problem. It will contain at least 2 grams of mercury, which is not enough. Even on government portals, in particular on mos.ru, there is an answer to the question of what to do in this case.
If you spill a small amount of mercury (a broken thermometer), first remove people and animals from the room, then open the window and close the door. It is also necessary to protect the respiratory system with a mask or bandage. After this, immediately begin collecting mercury.
It looks very harmless, but you shouldn't relax.
Two grams of mercury, which are contained in one thermometer, in a closed room with a volume of 20 cubic meters create a vapor concentration that is thousands of times higher than the level safe for humans.
How to collect mercury
When collecting mercury, do not sweep it away with a broom under any circumstances, so as not to provoke the formation of fine mercury dust. The larger the drops, the better. A vacuum cleaner is also a bad help, since the evaporated mercury will pass through the filters and end up in the air in an even more dangerous form.
A small amount of mercury leaked from a thermometer can be collected using an ordinary medical bulb or a sheet of paper and a knitting needle or thick needle. Pieces of plaster can be used to collect the smallest drops.
Put everything you collected and what you used to collect it in a jar and close the lid tightly. After this, thoroughly clean the scene. A good option would be to wipe this area with a solution of manganese or a soap-soda solution. When you finish cleaning, be sure to wash your hands thoroughly with soap.
Wash your hands thoroughly after cleaning up mercury.
How to throw away a broken thermometer and the mercury from it
Never throw collected mercury down the garbage disposal or sewer. This may lead to a further risk of uncontrolled infection. Place the jar of collected waste on the balcony or in the garage and, as soon as possible, hand it over to the demercurization center for disposal.
How to understand that you have been poisoned by mercury
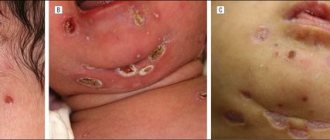
Symptoms of mercury poisoning take approximately 8 to 24 hours to appear. The main symptoms include general weakness, pain when swallowing, headache and fever. After some time, the gums and stomach begin to ache, and gastric disorders and even pneumonia appear. There have even been cases of death.
Skin cream can cause poisoning. How to avoid this?
Mercury in the atmosphere
According to experts, only up to 2,200 tons of mercury are released into the atmosphere annually from various anthropogenic sources (production of cement, steel, cast iron, non-ferrous metals; gold mining; waste disposal; operation of stationary furnaces). Its average content in the atmosphere ranges from 0.5 to 2.0 ng/m3 (MPC = 0.0003 mg/m3). The ratio of contributions of natural and anthropogenic sources to total air pollution depends on the specific region. Thus, the leader in global mercury emissions is Asia, accounting for 57% of mercury entering the atmosphere, followed by Europe (13%), Africa and North America (11% each), Australia and South America (5% and 3%). respectively). In the atmosphere, mercury is contained in approximately equal quantities in the form of vapors and in a state sorbed by aerosols.
In lightly polluted air, mercury concentrations are 0.8–1.2 ng/m3, in areas of large mercury deposits – up to 240 ng/m3, in areas of gas deposits – up to 70 thousand ng/m3. The mercury content in the air around enterprises (at a distance of up to 2 km) that produce or consume mercury can exceed the MPC by 4–5 times or more. At the same time, it has been shown that within a radius of 5 km from an organized emission source, no more than 6–10% of the total mercury emission occurs, and about 60% is transferred over distances of up to 100 km. As for methylmercury compounds, the most typical concentrations characterizing their content in the atmosphere range from 2 to 6 ng/m3. In St. Petersburg in 1991–1992. A survey of preschool institutions and schools was carried out to determine the content of mercury vapor in the air. Excesses of the maximum permissible concentration by tens and hundreds of times were registered in approximately every fourth preschool institution and every second school. It has been established that the reasons for such intense air pollution with mercury are the long-term, uncontrolled use of mercury devices, lamps, thermometers, and the like.
In Russia, the release of mercury into the air from industrial enterprises is approximately 10 tons per year. In principle, this is the level of most industrialized countries. In recent years, all over the world, including in the Russian Federation, active work has been going on to close the most harmful chlor-alkali production facilities. However, the problem of residual, overwhelmingly high levels of environmental pollution remains unresolved
Mercury in the lithosphere
The average content of inorganic mercury derivatives in the earth's crust is about 50 μg/kg. In soils, the natural content of mercury is usually assumed to average 10 ng/kg, but in contaminated areas the values of mercury concentrations can be two to three orders of magnitude higher. Various mercury compounds in the soil environment are in a state of dynamic equilibrium, in which the processes of methylation of inorganic mercury derivatives and demethylation of methylmercury compounds due to the presence of microorganisms play a significant role. The formation of methyl mercury derivatives leads to a significant increase in volatility.
Treatment methods for intoxication
In case of acute poisoning and chronic intoxication with mercury and its vapors, the patient is administered calcium gluconate, vitamins B6, B1. A solution of ascorbic acid and glucose is infused by infusion. In severe cases, taurine, succimer, unithiol or their analogs are used.
The main goal of these activities is to remove mercury from the human body. In addition to drug treatment, gastric lavage using a tube is used.
During the treatment process, the patient receives antiallergic medications and drugs that strengthen the victim’s immune system.
Inpatient treatment for mercury poisoning
When a poisoned person is admitted to a medical facility, he is diagnosed and suitable treatment and a set of procedures are selected, which are aimed at restoring all body functions and alleviating symptoms.
- An antidote is introduced.
- Repeated gastric lavage is allowed.
- The water-salt balance is corrected.
- Hemodialysis is used.
- In case of severe pain, a spinal cord block is performed.
- The patient is given diuretics.
- If the patient's condition worsens, the patient is transferred to intensive care.
- At the end, probiotics are introduced to restore the intestinal microflora.
Folk remedies
Traditional medicine recommends eating undercooked rice and red beets in case of mercury poisoning.
It is forbidden to eat spicy and salty foods, it is necessary to limit the consumption of fats.
Apple juice, which contains pectin, will help remove toxins. It is recommended to drink dairy products, a decoction of chamomile and alfalfa.
Mercury in living organisms
Mercury enters plants mainly through the atmosphere. Mercury coming from the atmosphere in the form of vapor is sorbed by coniferous plants and is firmly retained in the needles. Migration to other plant organs does not occur. According to the sanitary standards in force in the Russian Federation, the maximum permissible concentration for mercury compounds in agricultural plants (potatoes, vegetables, grains) is approved at the level of 0.02–0.03 mg/kg. At the same time, Russian studies have shown that organic and inorganic derivatives of mercury at such concentrations cause various negative ecotoxic effects in plants - inhibition of cellular respiration, decreased enzyme activity, etc.
One of the most striking examples of the effects of mercury on living organisms and humans is the notorious case of mass mercury poisoning in the Japanese city of Minamata. A special study found that Minamata disease is caused by the ecotoxicological effects of methylmercury compounds formed in aquatic ecosystems during the biological and chemical methylation of inorganic mercury derivatives. At the same time, the bioaccumulation of mercury compounds in marine biota reaches significant levels. In Minamata Bay, mercury concentrations were: in crabs - 35.7 mg/kg, in fish - 20.0 mg/kg, in shrimp - 5.6 mg/kg, compared to the Japanese standard of 0.4 mg/kg.
The fish of the Bratsk Reservoir (roach, crucian carp, bream and perch) also contain significant amounts of mercury - from 2 to 6 mg/kg, which, according to Siberian experts, is due to significant contamination of bottom sediments of this aquatic ecosystem with mercury and its compounds. According to the Environmental Protection Committee of the Irkutsk Region, industrial enterprises in Irkutsk, Angarsk, Usolye-Sibirsky and Zima are responsible for this pollution, some of which have discharged 1.5–2.0 thousand tons of wastewater over the past 20–30 years mercury
Freshwater fish (char, burbot and whitefish) in the Arctic waters of Russia contain mercury at a level of 0.01 µg/g wet weight (note for comparison that the corresponding values for the waters of Norway, Finland, Greenland and Canada are 0.25, 0.32 , 0.99 and 2.49 µg/g).
In Russian birds of prey, mercury levels are higher than in birds that eat only plant food. Mercury concentrations in seals and whales often exceed 0.5 µg/g muscle tissue (especially in older individuals). Of the highest values (so far obtained), noteworthy are the concentrations of mercury in the liver of ringed seals from the western regions of the Canadian Arctic (205 μg/g) and the liver of whales off the Faroe Islands (280 μg/g). As for polar bears, the mercury content in their fur ranges from 1.6–1.7 μg/g (at the mouth of the Lena River and on Wrangel Island) to 18.5 μg/g in Amundsen Bay (again off the northern coast of Canada , probably as a consequence of the existence of natural geological sources in this region). It should be noted that mercury bioaccumulation generally increases with temperature within normal physiological processes, as shown, for example, for shellfish.