Lamisil Cream for the treatment of foot fungus, cream 1% 30g
A country
Switzerland
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Terbinafine
Description
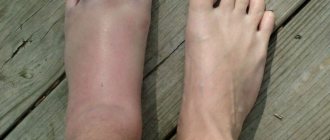
Lamisil cream is used to prevent and treat fungal skin infections, including athlete's foot.
The active substance of the cream Lamisil terbinafine has a fungicidal effect, with which it fights infection that causes fungus (mycosis) of the foot.* Suitable for use in adults and children over 12 years of age.* * Instructions for medical use, RU No. P N008851 dated 06/10/2010
Compound
Composition (per 1 g): Active ingredient mg: Terbinafine hydrochloride 10.0 Excipients: Sodium hydroxide* 1.2 Benzyl alcohol 10.0 Sorbitan stearate 19.0 Cetyl palmitate 20.0 Stearyl alcohol 40.0 Cetyl alcohol 40.0 Polysorbate-60 61.0 Isopropyl myristate 80.0 Purified water 718.8 *-Sodium hydroxide 30% solution by weight.
Product description
White homogeneous or almost homogeneous smooth cream with a weak characteristic odor.
pharmachologic effect
An antifungal drug for external use with a wide spectrum of antifungal activity. In small concentrations, terbinafine has a fungicidal effect against dermatophytes (Trychophyton rubrum, T.mentagrophytes, T.verrucosum, T.violaceum, T.tonsurans, Microsporum canis, Epidermophyton floccosum), yeast (mainly C.albicans) and certain dimorphic fungi ( Pityrosporum orbiculare or Mallassezia Furfur). Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic. Terbinafine specifically alters the early stage of sterol biosynthesis occurring in fungi. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine acts by inhibiting the enzyme squalene epoxidase located on the cell membrane of the fungus. Terbinafine does not affect the cytochrome P450 system in humans and, accordingly, the metabolism of hormones or other drugs. When used externally, absorption is less than 5% and has a slight systemic effect.
Indications for use
Treatment of fungal skin infections, including mycoses of the feet (“fungus” of the foot), tinea cruris, fungal infections of the smooth skin of the body (tinea corporis) caused by dermatophytes such as Trichophyton (including T. rubrum, T mentagrophytes, T. verrucosum, T. violaceum), Microsporum canis and Epidermophyton floccosum. Yeast infections of the skin, mainly those caused by the Candida genus (eg, Candida albicans), particularly diaper rash. Versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (also known as Malassezia furfur).
Contraindications
If you are hypersensitive to terbinafine or to any component of the drug; - During breastfeeding; - Children under 12 years of age due to insufficient data on effectiveness and safety.
Carefully
If you have one of the following diseases/conditions/risk factors, be sure to consult your doctor before taking the drug: - liver and/or kidney failure; - alcoholism; - inhibition of bone marrow hematopoiesis; - tumors; - metabolic diseases; - occlusive vascular diseases of the extremities; - pregnancy.
Use during pregnancy and lactation
Pregnancy In experimental studies, teratogenic properties of terbinafine were not identified. To date, no malformations have been reported with the use of Lamisil cream. Animal studies have not revealed any adverse effects on pregnancy or fetal health. However, since clinical experience with Lamisil cream in pregnant women is very limited, it should not be used unless absolutely necessary. During pregnancy, it is used only if the expected benefit to the mother outweighs the potential risk to the fetus. You should consult your doctor. Breastfeeding period Terbinafine is excreted in breast milk, so the drug should not be used by nursing mothers. The infant should not be allowed to come into contact with any surface of the skin treated with Lamisil cream.
Directions for use and doses
Externally. Adults and children over 12 years of age: Before applying the cream, clean and dry the affected areas. The cream is applied once or twice a day in a thin layer to the affected skin and surrounding areas and rubbed in lightly. For infections accompanied by diaper rash (under the mammary glands, in the spaces between the fingers, between the buttocks, in the groin area), the places where the cream is applied can be covered with gauze, especially at night. For extensive fungal lesions of the body, it is recommended to use the cream in tubes of 30 g. Average duration of treatment: - Dermatomycosis of the trunk, legs: 1 week, 1 time per day. — Dermatomycosis of the feet: 1 week, 1 time per day. — Skin candidiasis: 1-2 weeks, 1 or 2 times a day. — Lichen versicolor: 2 weeks, 1 or 2 times a day. A decrease in the severity of clinical manifestations is usually noted in the first days of treatment. If treatment is not regular or is stopped prematurely, there is a risk of the infection returning. If after one to two weeks of treatment there are no signs of improvement, the diagnosis should be verified. The dosage regimen for Lamisil cream in the elderly does not differ from that described above. Use of the drug in children. The use of this drug in children under 12 years of age is not recommended.
Side effect
Adverse reactions are listed below according to organ system classes and frequency of occurrence. The frequency of adverse reactions is determined as follows: very often (≥ 1/10); common (≥ 1/100 and Immune system disorders: Not known: hypersensitivity reactions (rash). Eye disorders: Rare: eye irritation. Skin and subcutaneous tissue disorders: Common: skin flaking, itching. Uncommon: skin damage, crusting, skin lesions, pigmentation disorder, erythema, burning sensation of the skin Rare: dry skin sensation, contact dermatitis, eczema Frequency unknown: rash General and injection site disorders: Uncommon: pain, pain at the application site , irritation at the site of application. Rarely: exacerbation of symptoms of the disease. At the site of application of the drug, itching, peeling of the skin, pain, irritation, changes in skin pigmentation, burning, erythema, crusts may be observed. These minor symptoms should be distinguished from hypersensitivity reactions such as rash occur in rare cases and require discontinuation of therapy. In rare cases, the course of a fungal infection may worsen. If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor
Overdose
No cases of drug overdose have been reported. Low systemic absorption of terbinafine for external use virtually eliminates overdose when applying the drug to the skin. Accidental ingestion of the contents of one 30 g tube of cream containing 300 mg terbinafine hydrochloride (equivalent to 264 mg terbinafine base) is comparable to taking one 250 mg terbinafine tablet (single oral dose for adults). Symptoms Signs of overdose after ingestion of terbinafine may include headache, nausea, epigastric pain and dizziness. Treatment Use of activated carbon, if necessary, symptomatic maintenance therapy. Further treatment should be carried out in accordance with clinical indications
Interaction with other drugs
No clinically significant drug interactions have been reported for Lamisil cream.
special instructions
A decrease in the severity of clinical manifestations is usually observed in the first days of treatment. In case of irregular treatment or its premature termination, there is a risk of recurrence of infection. Lamisil cream is for external use only. Avoid contact with eyes as it may cause irritation. If the drug accidentally gets into the eyes, they should be immediately rinsed with running water, and if persistent irritation develops, you should consult a doctor. If allergic reactions develop, the drug must be discontinued. Contact of infants with treated skin, including breasts, should be avoided. The drug contains cetyl and stearyl alcohols, which can cause local allergic reactions (contact dermatitis).
Release form
Cream for external use 1%. 7.5 g, 15 g or 30 g of the drug in an aluminum tube with a protective membrane made of aluminum or without a protective membrane made of aluminum, with a polypropylene cap equipped with a protrusion for perforating the membrane or without a protrusion for perforating the membrane or in a laminated tube (low polyethylene density, aluminum, low-density polyethylene) with a shoulder made of high-density polyethylene and with a protective membrane made of multilayer copolymer aluminum / ethylene, with a cover made of polypropylene, equipped with a projection for perforating the membrane. The tube with instructions for use is placed in a cardboard box. Secondary packaging is allowed to have a first-opening control.
Storage conditions
Store at a temperature not exceeding 30 ºC. Keep out of the reach of children.
Best before date
3 years. Do not use after expiration date.
Lamisil
Lamisil (INN terbinafine) is an antimycotic agent, a structural derivative of allylamine. It has a wide therapeutic range, allowing the drug to be used for diseases of the skin, hair and nails caused by various pathogens, including trichophyton, mycospores, epidermophyton, candida. In relation to molds and dermatophytes, small concentrations of terbinafine are sufficient to achieve a fungicidal effect. For yeast fungi, the type of pharmacological activity (fungicidal or fungistatic) largely depends on the concentration of the active substance. Terbinafine, used in Lamisil as an active ingredient, is one of the most effective antimycotic substances today. In terms of the severity of its fungicidal (fungistatic) action, it is superior to other antifungal drugs: imidazole derivatives, first generation triazoles, polyene antibiotics, echinocandins. The greatest sensitivity to the action of the drug is shown by trichophytons, mycospores, epidermophytons (maximum - pathogenic mold fungi and red trichophyton), slightly less - aspergillus, sporothrix and fungi of the genus Candida. Lamisil's key competitive advantages stem from its pharmacokinetic characteristics. After entering the digestive tract, the maximum concentration in the blood serum of the active component of the drug is established within 2 hours. The lipophilicity of terbinafine ensures a high rate of absorption through the dermis and accumulation in the stratum corneum and nails. The ability of the substance to be secreted with sebum creates its high concentrations in the hair and hair follicles. Sustained therapeutic concentrations of terbinafine can be achieved within 10-14 days of oral administration. The half-life of the drug varies widely from 25 to 155 days. In nail plates, Lamisil can remain in therapeutic concentrations for up to 1 year after cessation of drug therapy.
The degree of its absorption in the gastrointestinal tract is not affected by the pH of gastric juice, which plays an important role in the treatment of elderly patients, whose share in the overall structure of the incidence of mycoses is 40%. A feature of Lamisil is also its minimal interaction with commonly used drugs: antidiabetic, antiarrhythmic, calcium channel blockers, corticosteroids, sedatives, etc., which minimizes the negative consequences of polypharmacy. Another undeniable advantage of the drug is its favorable safety profile: Lamisil has a minimal list of contraindications for use. Among all modern systemic antifungal drugs, Lamisil is one of the safest. In clinical trials LION. (1999) and LION.IES (2000), conducted according to all the rules of evidence-based medicine, the drug was compared with another systemic antimycotic agent - itraconazole. As a result of the research, it was found that the effectiveness of regular pharmacotherapy with Lamisil was twice as high as pulse treatment with itraconazole. The second of the above studies assessed the effectiveness of Lamisil treatment over a five-year period. As a result, it was found that cure in the Lamisil group occurred 3.5 times more often than in patients receiving pulse therapy with itraconazole, while in the latter group relapses were 2.5 times more likely. To date, research on Lamisil has not yet been completed: scientists believe that the capabilities of this drug are much wider than those listed in the instructions for use. Currently, optimal therapeutic algorithms for using the drug for systemic mycoses and onychomycosis in people with diabetes are being studied.